Successful Usage of Extracorporeal Plasma Perfusion Adsorption Devices Columns for Desensitization Through Immunoadsorption in End-Stage Renal Disease Patients for ABO-Incompatible Kidney Transplant
DOI:
https://doi.org/10.14740/wjnu1017Keywords:
ABO-incompatible transplant, SECORIM immunoadsorption column desensitization, Tacrolimus, Isoagglutinin titers, Kidney transplantationAbstract
Background: ABO-incompatible kidney transplantation (ABOiKT) has emerged as a viable solution to overcome donor shortages, particularly in countries with underdeveloped deceased donor programs. This study evaluated the efficacy of the SECORIM ABO immunoadsorption (IA) column (Vitrosorb AB, Malmo, Sweden) desensitization protocol in 21 end-stage renal disease (ESRD) patients undergoing live donor ABOiKT.
Methods: Patients underwent rituximab induction (375 - 500 mg), followed by individualized IA (1 - 3 sessions) using SECORIM ABO columns. Pre-transplant isoagglutinin IgG titers ranged from 1:32 to 1:1,024, successfully reduced to ≤ 1:8 before transplantation. Post-operative immunosuppression included tacrolimus, mycophenolate mofetil, and corticosteroids.
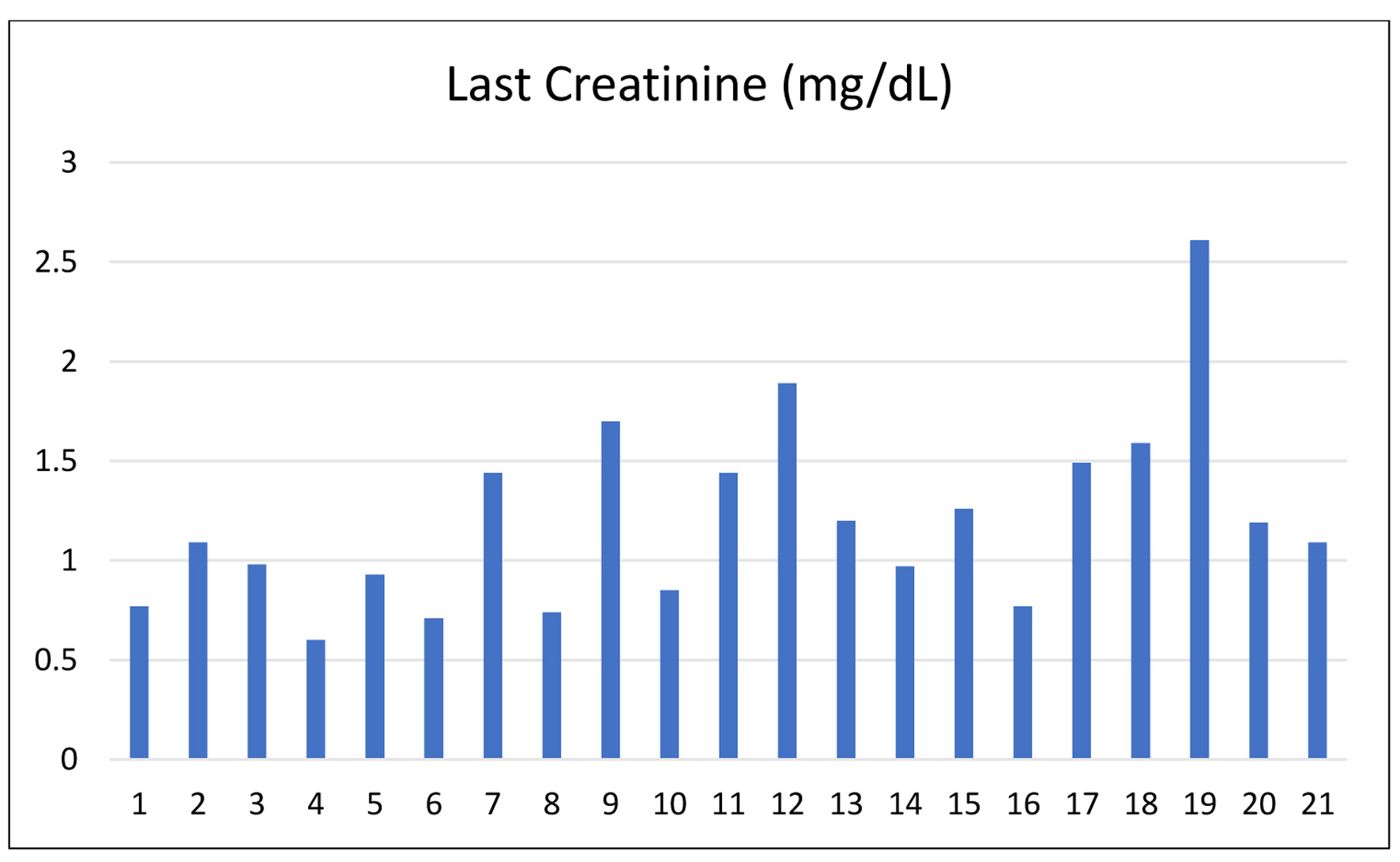
Results: All recipients demonstrated stable graft function with no early rejection. The mean serum creatinine at discharge was 1.2 mg/dL (range 0.6 - 2.61 mg/dL), and tacrolimus trough levels varied between 6.19 and 24.9 ng/mL. There were no incidences of hyperacute rejection or graft loss.
Conclusion: The IA protocol proved effective in facilitating safe ABOiKT, ensuring optimal immunological modulation with favorable short-term outcomes, offering a reproducible framework for resource-constrained healthcare settings.
Published
Issue
Section
License
Copyright (c) 2025 The authors

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.





