| World Journal of Nephrology and Urology, ISSN 1927-1239 print, 1927-1247 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Nephrol Urol and Elmer Press Inc |
| Journal website https://wjnu.elmerpub.com |
Original Article
Volume 14, Number 2, November 2025, pages 35-43
Successful Usage of Extracorporeal Plasma Perfusion Adsorption Devices Columns for Desensitization Through Immunoadsorption in End-Stage Renal Disease Patients for ABO-Incompatible Kidney Transplant
Vikram Kalraa, c, Anupam Roya, Umesh Guptaa, Ambar Khairab
aDepartment of Nephrology and Renal Transplant, Aakash Healthcare Super Speciality Hospital, New Delhi, India
bUniversity of Western Ontario, London Health Sciences Centre (LHSC), London, Ontario, Canada
cCorresponding Author: Vikram Kalra, Department of Nephrology and Renal Transplant, Aakash Healthcare Super Speciality Hospital, New Delhi, India
Manuscript submitted September 25, 2025, accepted October 18, 2025, published online November 8, 2025
Short title: ECPPAD for ABOiKT in High-Titer ESRD
doi: https://doi.org/10.14740/wjnu1017
| Abstract | ▴Top |
Background: ABO-incompatible kidney transplantation (ABOiKT) has emerged as a viable solution to overcome donor shortages, particularly in countries with underdeveloped deceased donor programs. This study evaluated the efficacy of the SECORIM ABO immunoadsorption (IA) column (Vitrosorb AB, Malmo, Sweden) desensitization protocol in 21 end-stage renal disease (ESRD) patients undergoing live donor ABOiKT.
Methods: Patients underwent rituximab induction (375 - 500 mg), followed by individualized IA (1 - 3 sessions) using SECORIM ABO columns. Pre-transplant isoagglutinin IgG titers ranged from 1:32 to 1:1,024, successfully reduced to ≤ 1:8 before transplantation. Post-operative immunosuppression included tacrolimus, mycophenolate mofetil, and corticosteroids.
Results: All recipients demonstrated stable graft function with no early rejection. The mean serum creatinine at discharge was 1.2 mg/dL (range 0.6 - 2.61 mg/dL), and tacrolimus trough levels varied between 6.19 and 24.9 ng/mL. There were no incidences of hyperacute rejection or graft loss.
Conclusion: The IA protocol proved effective in facilitating safe ABOiKT, ensuring optimal immunological modulation with favorable short-term outcomes, offering a reproducible framework for resource-constrained healthcare settings.
Keywords: ABO-incompatible transplant; SECORIM immunoadsorption column desensitization; Tacrolimus; Isoagglutinin titers; Kidney transplantation
| Introduction | ▴Top |
End-stage renal disease (ESRD) represents a growing global health burden, with kidney transplantation regarded as the definitive therapeutic modality that offers superior survival and enhanced quality of life over dialysis. However, the widening gap between the demand and availability of suitable organ donors, particularly in regions where deceased donor programs are underdeveloped, continues to pose a critical challenge. In countries like India, where live-related kidney donation predominates, ABO blood group incompatibility has historically been a major immunologic barrier, significantly limiting donor options and exacerbating waitlist mortality [1, 2].
ABO-incompatible kidney transplantation (ABOiKT) has emerged as a transformative approach to overcome this immunological hurdle, effectively expanding the donor pool. Despite early experiences with high rates of antibody-mediated rejection (AMR) and graft loss, advancements in desensitization strategies have substantially improved outcomes, making ABOiKT a viable alternative to ABO-compatible transplantation [3, 4]. These advancements primarily focus on mitigating hyperacute rejection caused by preformed anti-ABO isoagglutinins, which are naturally occurring antibodies capable of eliciting a potent immune response against incompatible grafts [5].
The evolution of desensitization protocols incorporating rituximab, plasmapheresis, intravenous immunoglobulin (IVIG), and, more recently, antigen-specific immunoadsorption (IA) has been pivotal in achieving successful ABOiKT. Rituximab, a monoclonal antibody targeting CD20+ B cells, plays a central role in reducing the production of isoagglutinins, thereby minimizing humoral immunity against the graft [6, 7]. Simultaneously, IA methods using antigen-specific columns have improved the selective elimination of antibodies while conserving vital plasma constituents [8].
The IA column protocol integrates these innovations into a streamlined, resource-efficient desensitization regimen (Table 1). By combining rituximab-mediated B-cell depletion with targeted IA using an IA column and adjunctive hemodialysis (HD) sessions for metabolic optimization, the IA column offers a simplified yet effective approach to ABOiKT desensitization. Unlike traditional protocols that depend on multiple plasmapheresis sessions and high-dose IVIG, IA offers a more efficient approach by reducing procedural burden, minimizing non-specific plasma loss, and lowering overall cost factors that are especially critical in low- to middle-income countries [9-11].
 Click to view | Table 1. Overview of Immunological Strategy in ABOiKT |
Numerous studies have validated the efficacy of rituximab-based desensitization in preventing AMR, with 1-year patient and graft survival rates exceeding 90%, paralleling outcomes seen in ABO-compatible transplants [12-14]. Additionally, antigen-specific IA has demonstrated superior efficacy in isoagglutinin reduction with fewer sessions and lower complication rates compared to conventional plasmapheresis [15, 16]. The IA approach further refines these principles, promoting a modified, patient-centric desensitization model adaptable to various clinical settings.
This study presents a retrospective case series of 21 patients who underwent ABOiKT using the IA desensitization protocol at a tertiary care center in India. The primary aim was to assess the efficacy of IA in achieving adequate isoagglutinin titer reduction, ensuring stable early graft function, and maintaining clinical safety across diverse donor-recipient pairs. By documenting clinical outcomes such as serum creatinine levels, tacrolimus trough concentrations, and absence of rejection episodes, this study underscores the clinical relevance and reproducibility of the IA protocol, advocating for its broader adoption in resource-constrained healthcare environments.
| Materials and Methods | ▴Top |
The renal transplant cases were retrospectively observed at a tertiary care transplant center in India between March 2021 and July 2024. The study enrolled 21 patients diagnosed with ESRD who underwent live donor ABOiKT. All patients received kidneys from first-degree relatives with incompatible ABO blood groups, necessitating pre-transplant desensitization. Informed consent was obtained from all patients before the initiation of desensitization and surgical procedures.
Pre-transplant evaluation
Each patient underwent a rigorous pre-transplant assessment, which included a detailed clinical history, physical examination, and comprehensive laboratory workup. The workup included complete blood count (CBC), renal function tests (RFTs), liver function tests (LFTs), coagulation profile, and viral serologies (human immunodeficiency virus (HIV), hepatitis B surface antigen (HBsAg), and hepatitis C virus (HCV)). Blood group determination was confirmed via standard agglutination methods. Crucially, isoagglutinin titers (both IgG and IgM classes) against incompatible ABO antigens were measured using column agglutination technology. Human leukocyte antigen (HLA) matching and lymphocyte crossmatching were also performed to evaluate immunological compatibility.
Desensitization protocol: IA approach
Desensitization was initiated 2 - 3 weeks before the planned transplant using the IA protocol. This protocol integrated rituximab-based B-cell depletion with targeted antigen-specific IA and adjunctive HD, streamlining the desensitization process. The SECORIM® IA column setup used during desensitization is shown in Figure 1, illustrating the dual-column filtration system and plasma circulation pathway for antibody removal.
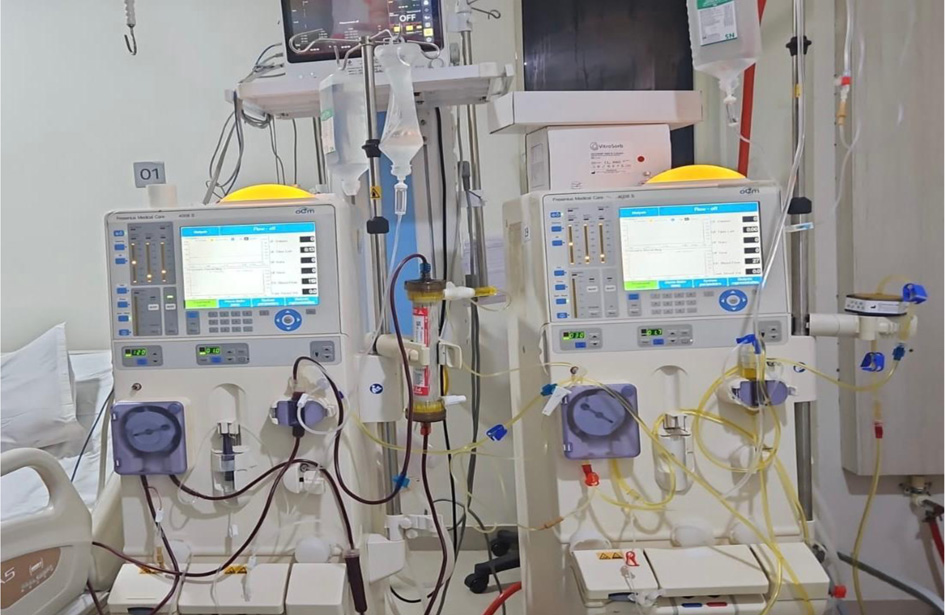 Click for large image | Figure 1. SECORIM® immunoadsorption column setup. |
All patients received a single intravenous dose of rituximab (375 - 500 mg) 2 weeks before transplantation. Depending on baseline isoagglutinin titers, patients underwent 1 - 3 sessions of IA therapy using IA columns, which selectively removed anti-A and anti-B antibodies without depleting essential plasma proteins. HD was employed as necessary to manage fluid overload, correct metabolic derangements, and enhance antibody removal. The target was to achieve anti-ABO IgG titers of ≤ 1:8 before proceeding to transplantation.
Unlike conventional desensitization protocols, which are heavily dependent on plasmapheresis and IVIG, IA employed a simplified yet highly selective approach, reducing the procedural burden and plasma loss. This methodology proved particularly advantageous in resource-constrained settings by offering a cost-effective and efficient desensitization strategy without compromising immunologic efficacy.
Transplant procedure
All 21 renal transplants were performed by the same surgical team using a standardized extra-peritoneal approach under general anesthesia. The donor kidney was anastomosed end-to-side to the recipient’s external iliac artery and vein. Ureteroneocystostomy was performed with stenting based on intraoperative findings. The intraoperative course was uneventful in all cases, with satisfactory graft perfusion confirmed by Doppler assessment.
Immunosuppression and post-operative monitoring
The post-transplant immunosuppressive regimen included: 1) tacrolimus (starting dose: 0.1 mg/kg/day in two divided doses), titrated to maintain trough levels between 8 and 12 ng/mL; 2) mycophenolate sodium (720 mg/day); 3) oral prednisolone, initiated at 20 mg/day and tapered; and 4) anti-thymocyte globulin (ATG) induction (1 - 2 mg/kg) administered in all patients in view of high immunologic risk patients.
Patients were monitored intensively in the immediate post-operative period with daily assessments of urine output, hemodynamic stability, serum creatinine levels, and tacrolimus trough concentrations. Anti-A and/or anti-B isoagglutinin titers were rechecked post-operatively in selected patients to monitor for rebound responses.
Follow-up and outcome assessment
Patients were followed for 3 to 42 months. During the first month, weekly follow-ups included CBC, RFT, LFT, and tacrolimus monitoring. Doppler ultrasonography was performed within 1 week of transplantation and subsequently as required. Long-term surveillance involved periodic assessment of renal function, drug levels, and screening for infectious or immunological complications.
Outcomes
All 21 patients were successfully transplanted, with no episodes of hyperacute or biopsy-proven acute rejection during follow-up. No graft loss or severe infectious complications were documented. At discharge, all patients demonstrated stable renal function, with serum creatinine values ranging from 0.6 to 2.61 mg/dL.
Institutional Review Board (IRB) approval
The study protocol was reviewed and approved by the Institutional Ethics Committee of Aakash Healthcare Super Speciality Hospital, New Delhi.
Ethical compliance with human study
This study was conducted in compliance with the ethical standards of the responsible institutional and national committees on human experimentation and with the principles of the Helsinki Declaration.
| Results | ▴Top |
Demographics, desensitization protocol, and post-transplant outcomes
A total of 21 patients with chronic kidney disease (CKD) stage 5 underwent ABOiKT following a rituximab-based desensitization protocol. The group consisted of 15 males and six females with a mean age of 42.5 years (range, 28 - 61 years). All recipients received grafts from living-related donors. Parental donations were the most common donor category (42.8%), followed by spousal donations (33.3%), siblings (19.0%), and other relatives (4.7%). Regarding blood groups, recipients were predominantly O+ (47.6%) and B+ (33.3%) and A+ (19.0%), whereas donors were most commonly A+ (38%), reflecting the inherent ABO incompatibility. The detailed demographic and clinical features, including age, gender, donor relationship, blood groups, and transplant dates, are presented in Table 2. All patients successfully underwent desensitization, ensuring safe transplantation despite blood group barriers.
 Click to view | Table 2. Demographic and Clinical Parameters at Discharge |
The desensitization strategy (Table 3) involved rituximab administration approximately 2 weeks before transplantation, followed by IA using ABO-specific columns. Each patient required between one and three IA sessions, with two sessions being most common (66.6%).
 Click to view | Table 3. Desensitization Protocol and Isoagglutinin Titers |
Pre-desensitization IgG titers demonstrated wide variability, from as low as 1:32 to as high as 1:1,024, indicating heterogeneous baseline sensitization. Following the protocol, most patients achieved substantial reductions in antibody levels, with post-IgG titers reduced to ≤ 1:8 in the majority. Patient 1 achieved complete clearance (Nil), while most patients successfully reduced titers to 1:2 - 1:4. However, patients 5, 8, and 14 retained titers of 1:8, underscoring the variability in individual responses (Figs. 2 and 3).
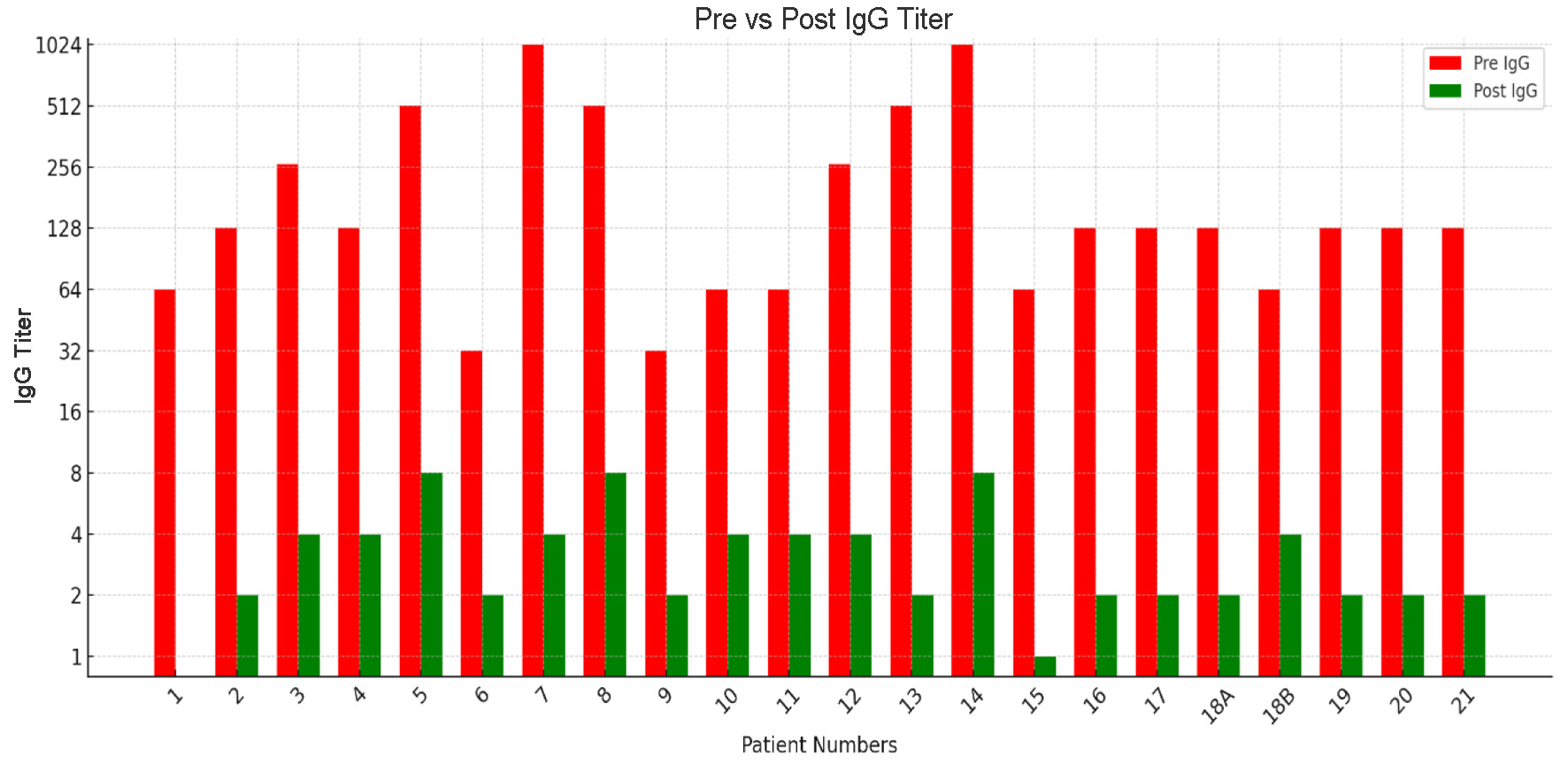 Click for large image | Figure 2. Pre- and post-desensitization IgG isoagglutinin titers across patients. |
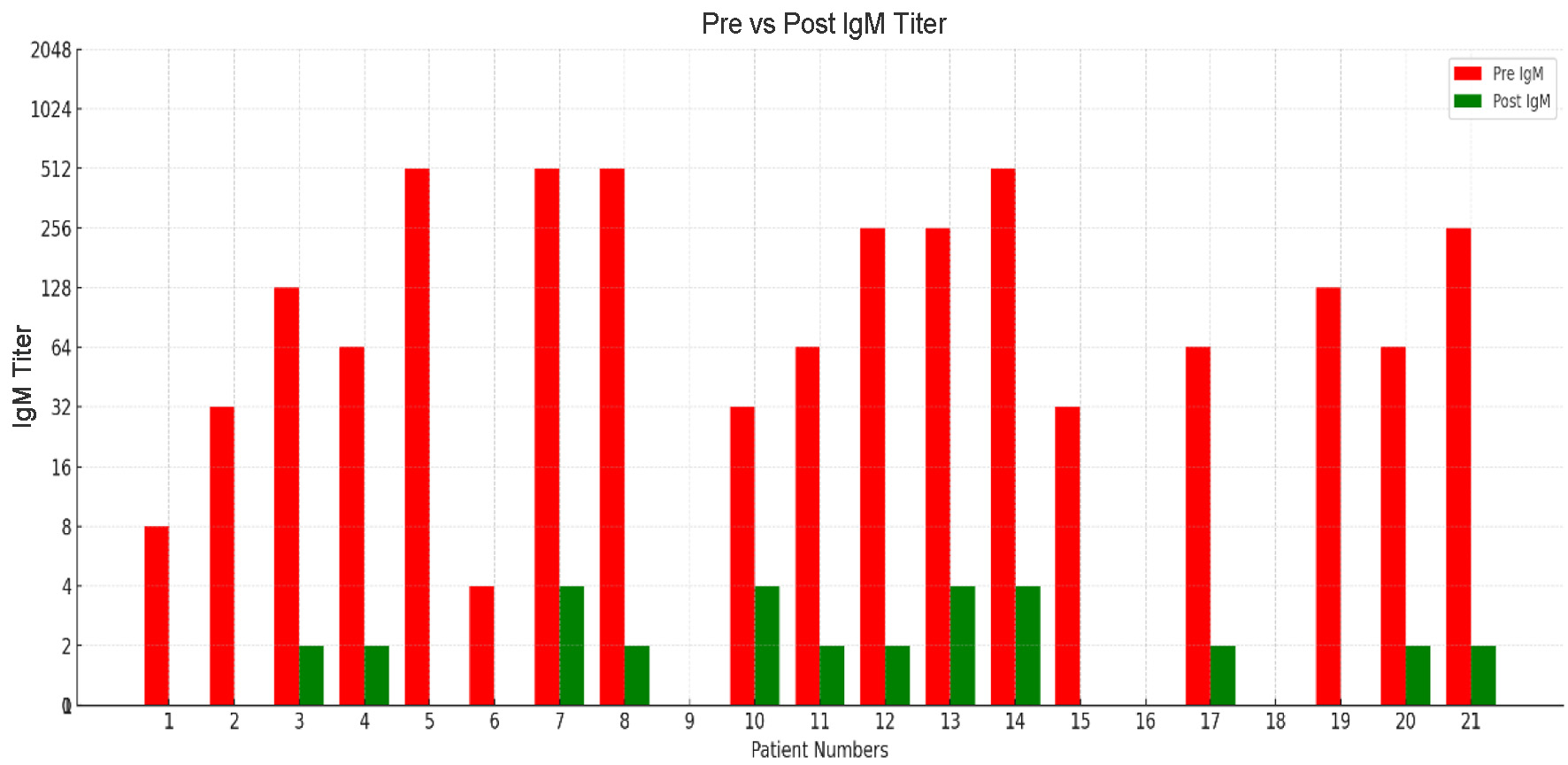 Click for large image | Figure 3. Pre- and post-desensitization IgM isoagglutinin titers across patients. |
Similarly, IgM titers, which often reflect early antibody-mediated activity, showed favorable declines. While pre-treatment titers were higher in several patients, most achieved a significant reduction to 1:2 - 1:8 before surgery. This decline in IgM is clinically important, as uncontrolled IgM levels may accelerate graft rejection. Overall, the protocol effectively reduced both IgG and IgM titers, ensuring safe progression to ABO-incompatible transplantation, though certain patients required more intensive therapy.
Table 4 provides an overview of post-transplant graft function indicators among 21 patients, focusing on discharge creatinine and tacrolimus levels. The number of hospitalization days ranged from as short as 6 days to as long as 21 days, reflecting variations in individual recovery and clinical needs. Creatinine, a key marker of renal function, was within normal or near-normal limits for the majority of patients, ranging between 0.6 and 1.7 mg/dL. Notably, patients 12 and 19 presented with relatively higher values of 1.89 and 2.61 mg/dL, respectively, suggesting possible mild graft dysfunction that warranted closer monitoring. In contrast, most patients maintained stable creatinine values under 1.5 mg/dL, indicating satisfactory graft performance (Fig. 4).
 Click to view | Table 4. Post-Transplant Graft Function Indicators: Creatinine and Tacrolimus Levels at Discharge |
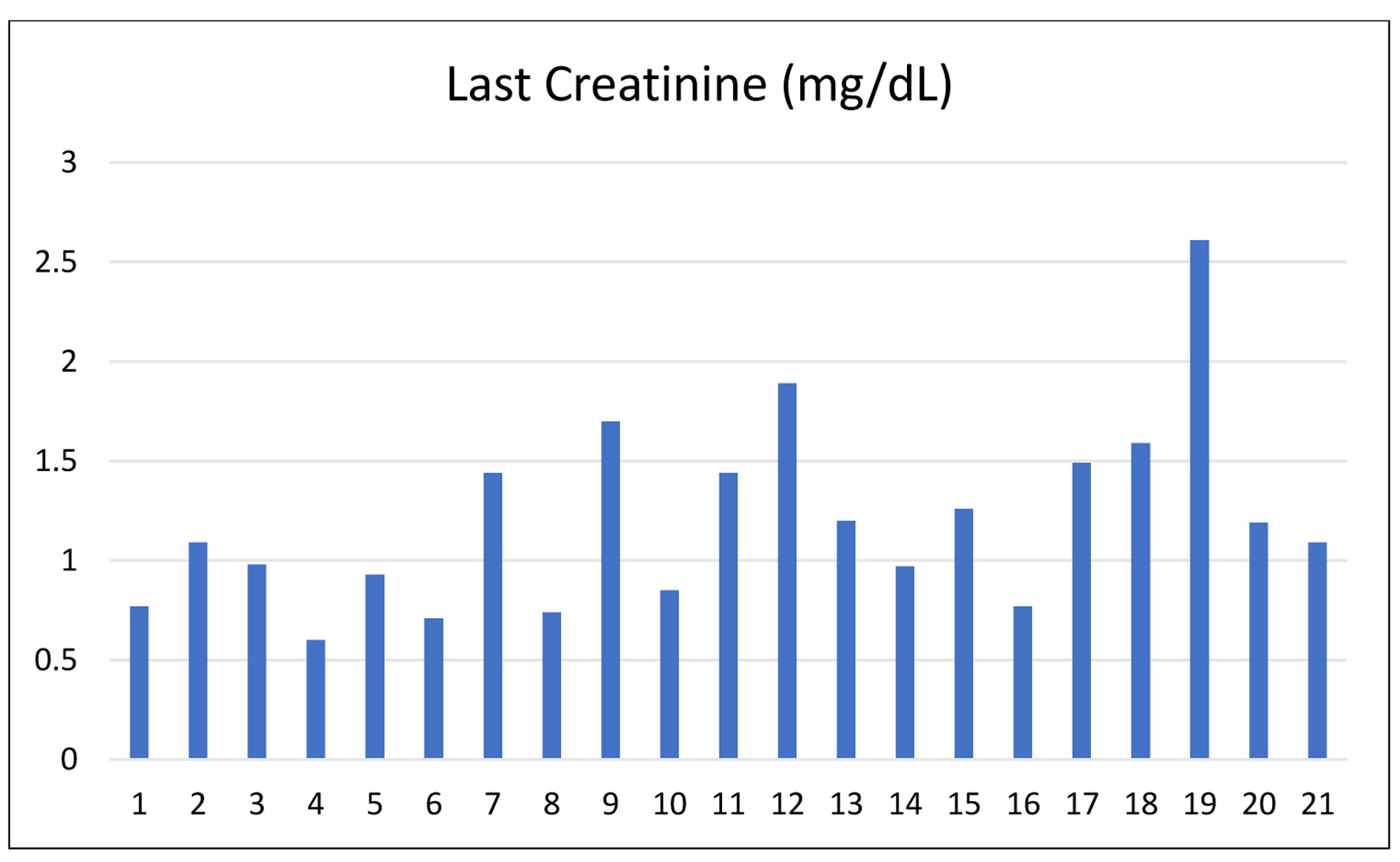 Click for large image | Figure 4. Last serum creatinine levels at discharge across patients. |
Tacrolimus levels, critical for maintaining immunosuppression and preventing rejection, displayed wide interpatient variation, ranging from 6.19 to 24.9 ng/mL. While most patients remained within the therapeutic window of 6 - 16 ng/mL, some patients (2, 14, 15, and 21) showed higher levels (> 20 ng/mL), likely reflecting early post-transplant intensive immunosuppression regimens (Fig. 5).
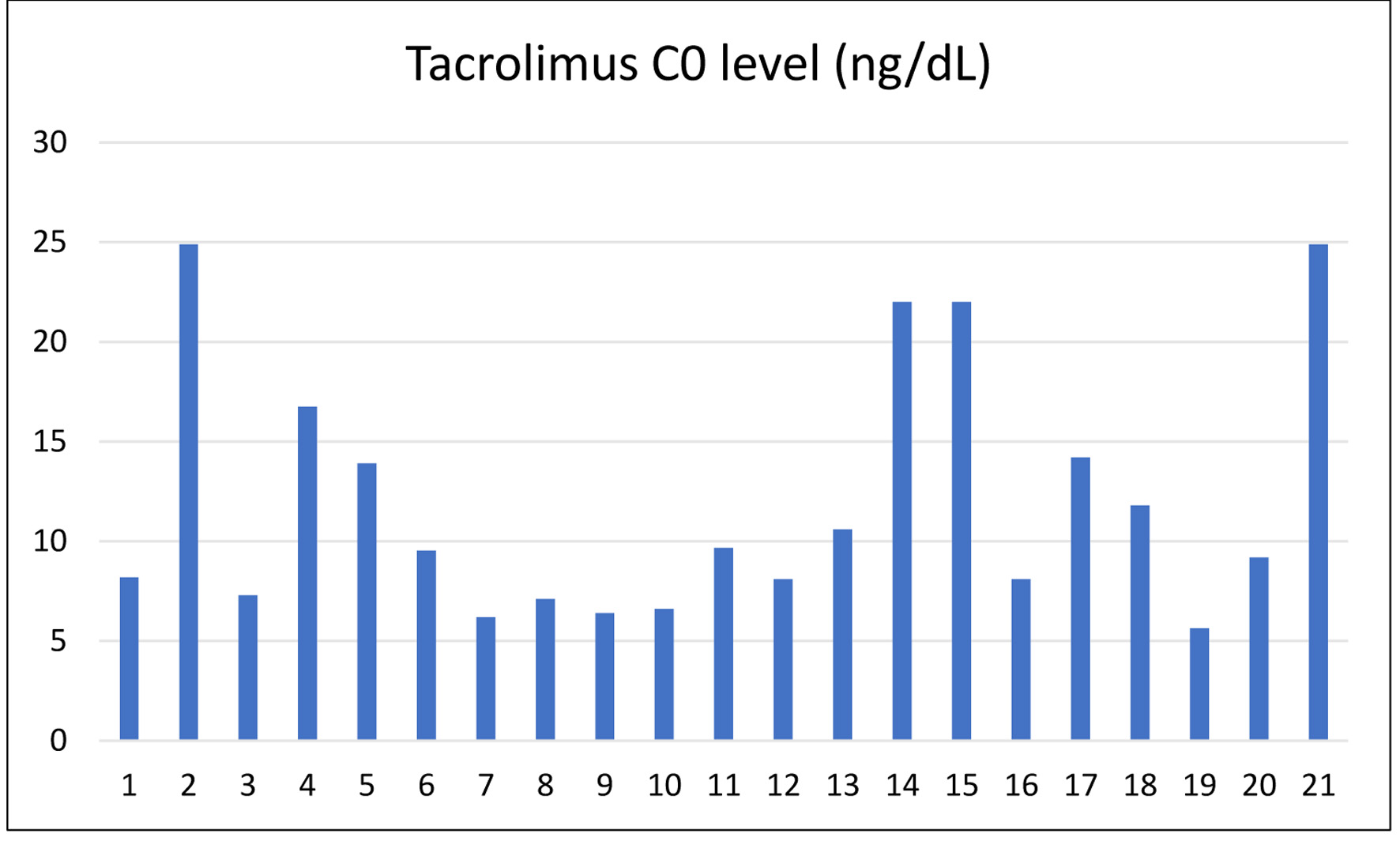 Click for large image | Figure 5. Last tacrolimus levels at discharge across patients. |
To summarize, the IA-guided desensitization protocol successfully reduced anti-ABO antibody titers to permissible levels in most patients, facilitating successful transplantation outcomes. Intensive IA and HD strategies were particularly crucial for patients presenting with high baseline titers. The post-transplant graft function indicators suggest that tailored desensitization protocols, even under resource constraints, can achieve favorable outcomes in ABOiKT recipients.
| Discussion | ▴Top |
The use of ABOiKT has transformed the field of kidney transplantation by enabling the use of a wider pool of living donors. Previously, the existence of preformed anti-A/B antibodies was a major stumbling block towards successful transplantation based on the threat of hyperacute and antibody-driven rejection. With the advent of advanced desensitization protocols, the threat of rejection has been eliminated, making ABOiKT a feasible option even in low-resource situations. Rituximab has been instrumental in desensitization protocols, being successful in depleting CD20+ B cells and thus decreasing the generation of anti-ABO antibodies [17]. Combination with antigen-specific IA via IA columns enables specific removal of isoagglutinins and is linked with fewer complications than standard plasmapheresis [18].
This group of 21 patients who received live donor ABOiKT at a tertiary care facility in India highlights the clinical efficacy and feasibility of standardized desensitization protocols. The IA column protocol was pivotal in this success by offering a systematic, tiered strategy of immunological preparation. IA brings together rituximab-mediated B-cell depletion with focused IA/plasmapheresis to achieve a further decline in isoagglutinin titers before transplant. Among the study, only one patient required post-transplant biopsy due to surgical re-exploration, showing partial cortical necrosis.
Procedural outcomes were significantly favorable, with all recipients having stable graft function at discharge. The lack of biopsy-proven rejection or premature graft loss is in line with recent reports emphasizing the efficacy of rituximab-based protocols. In addition, in spite of differences in baseline immunologic risk profiles as indicated by conflicting pre-treatment titers, clinical outcomes were consistent across the board. This confirms the malleability of the IA column to patient-specific needs [19, 20].
Serum creatinine levels at discharge were within safe ranges, and tacrolimus levels were carefully managed to maximize immunosuppression without nephrotoxicity. The broad variability of tacrolimus trough levels noted highlights the need for personalized dosing, particularly in the setting of variable IA needs and metabolic tolerance. The strategic addition of IA enhanced procedural predictability by normalizing pre-transplant immunologic modulation. In contrast to protocols dependent on plasmapheresis alone, IA column-based IA allowed for selective and target antibody removal with fewer procedural burdens and complications.
Yet, there were problems. One patient had delayed graft function due to intraoperative issues and required re-exploration. Notwithstanding this, eventual stabilization was evidence of the robustness of the protocol even under complex situations. Such situations underscore the importance of close perioperative surveillance and individualized post-operative care protocols. This experience is consistent with international data that reported 1-year graft survival rates of more than 90% in well-organized ABOiKT programs. It also makes IA an applicable, replicable model, which is specifically useful in areas where deceased donor programs are immature [21-27].
Conclusion
The clinical cases described here offer strong evidence that ABOiKT, supported by the IA desensitization protocol, is an effective and safe treatment for patients with ESRD threatened by donor incompatibility. The protocol was effective in overcoming immunologic barriers to allow successful engraftment without early rejection or graft loss in varied recipient-donor relationships. The clinical effectiveness of this regimen was reflected in the consistently positive short-term results, characterized by stable serum creatinine concentrations, tolerably well-treated immunosuppression protocols, and freedom from episodes of acute rejection. The inclusion in the IA protocol of rituximab induction with selective IA demonstrates a sophisticated and highly responsive desensitization process amenable to use in developing regions.
Acknowledgments
The authors sincerely acknowledge the entire Nephrology and Urology transplant teams, laboratory staff, and nursing personnel for their unwavering support in patient care and data collection. Special thanks to the patients and their families for their trust and consent to share their medical records for academic purposes.
Financial Disclosure
No specific funding was received from public, commercial, or not-for-profit sectors for this study. The research was conducted as part of routine clinical practice.
Conflict of Interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
Informed Consent
Written informed consent was obtained from all individual participants included in this study.
Author Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Dr Vikram Kalra, Dr Anupam Roy, Dr Umesh Gupta, and Dr Ambar Khaira. Dr Vikram Kalra wrote the first draft of the manuscript, and all authors commented on previous versions. All authors read and approved the final manuscript.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
| References | ▴Top |
- Hariharan S, Johnson CP, Bresnahan BA, Taranto SE, McIntosh MJ, Stablein D. Improved graft survival after renal transplantation in the United States, 1988 to 1996. N Engl J Med. 2000;342(9):605-612.
doi pubmed - Resnick MI. Urolithiasis-foreword. Urologic Clinics of North America. 2007;34(3):XIII.
- Montgomery RA, Locke JE, King KE, Segev DL, Warren DS, Kraus ES, Cooper M, et al. ABO incompatible renal transplantation: a paradigm ready for broad implementation. Transplantation. 2009;87(8):1246-1255.
doi pubmed - D'Cunha PT, Parasuraman R, Venkat KK. Rapid resolution of proteinuria of native kidney origin following live donor renal transplantation. Am J Transplant. 2005;5(2):351-355.
doi pubmed - Floege J, Mak RH, Molitoris BA, Remuzzi G, Ronco P. Nephrology research—the past, present and future. Nat Rev Nephrol. 2015;11(11):677-687.
doi pubmed - Genberg H, Hansson A, Wernerson A, Wennberg L, Tyden G. Pharmacodynamics of rituximab in kidney allotransplantation. Am J Transplant. 2006;6(10):2418-2428.
doi pubmed - Montgomery RA, Lonze BE, King KE, Kraus ES, Kucirka LM, Locke JE, Warren DS, et al. Desensitization in HLA-incompatible kidney recipients and survival. N Engl J Med. 2011;365(4):318-326.
doi pubmed - Almirall J, Comas L, Martinez-Ocana JC, Roca S, Arnau A. Effects of chronotherapy on blood pressure control in non-dipper patients with refractory hypertension. Nephrol Dial Transplant. 2012;27(5):1855-1859.
doi pubmed - Tanabe K. Japanese experience of ABO-incompatible living kidney transplantation. Transplantation. 2007;84(12 Suppl):S4-7.
doi pubmed - Takahashi K, Saito K. ABO-incompatible kidney transplantation. Transplant Rev (Orlando). 2013;27(1):1-8.
doi pubmed - Tiwari AK, Aggarwal G, Arora D, Bhardwaj G, Jain M, Bansal SB, Sethi SK. Immunoadsorption in ABO-incompatible kidney transplantation in adult and pediatric patients with follow-up on graft and patient survival: First series from India. Asian J Transfus Sci. 2020;14(1):13-18.
doi pubmed - Aikawa A, Saito K, Takahashi K. Trends in ABO-incompatible kidney transplantation. Exp Clin Transplant. 2015;13(Suppl 1):18-22.
pubmed - Tyden G, Kumlien G, Fehrman I. Successful ABO-incompatible kidney transplantations without splenectomy using antigen-specific immunoadsorption and rituximab. Transplantation. 2003;76(4):730-731.
doi pubmed - Prabhakar A, Gang S, Hegde U, Konnur A, Patel H, Rajapurkar M. Kidney transplantation with ABO-incompatible donors: a comparison with matched ABO compatible donor transplants. Indian J Nephrol. 2021;31(4):358-364.
doi pubmed - Rydberg L. ABO-incompatibility in solid organ transplantation. Transfus Med. 2001;11(4):325-342.
doi pubmed - Agrawal S, Chowdhry M, Makroo RN, Nayak S, Gajulapalli SP, Thakur UK, Agrawal A. Therapeutic Immunoadsorption and Conventional Plasma Exchange in ABO-incompatible Renal Transplant: An Exculpatory Evidence. Cureus. 2019;11(5):e4787.
doi pubmed - Marshall S, Tsveybel K, Boukedes S, Chepuri R, Coppolino A, El-Chemaly S, Hartigan P, et al. Limited effect of prevention strategies on incidence of clinically detectable venous thromboembolism after lung transplantation. Transplant Proc. 2023;55(9):2191-2196.
doi pubmed - Orandi BJ, Luo X, Massie AB, Garonzik-Wang JM, Lonze BE, Ahmed R, Van Arendonk KJ, et al. Survival Benefit with Kidney Transplants from HLA-Incompatible Live Donors. N Engl J Med. 2016;374(10):940-950.
doi pubmed - Gloor JM, Stegall MD. ABO incompatible kidney transplantation. Curr Opin Nephrol Hypertens. 2007;16(6):529-534.
doi pubmed - Kinsey GR, Okusa MD. Pathogenesis of acute kidney injury: foundation for clinical practice. Am J Kidney Dis. 2011;58(2):291-301.
doi pubmed - Braat AE, Blok JJ, Rahmel AO, Adam R, Burroughs AK, Putter H, Porte RJ, et al. Incorporation of donor risk into liver allocation algorithms. Am J Transplant. 2013;13(2):524-525.
doi pubmed - Kalra V, Jain S, Anand Y, Khaira A, Gupta U. Clinical evidence of immunoadsorption column for desensitization in ABO-incompatible renal transplantation (ABOi-RTx) patients: a case series. J Clin Exp Nephrol. 2023;8(4):205.
- Mahajan S, Tiwari SC, Kalra V, Masih JA, Bhowmik DM, Bansal R, Agarwal SK. Analysis of depression and its effect on outcome among adult Indian peritoneal dialysis patients. Perit Dial Int. 2007;27(1):94-96.
pubmed - Kalra V, Agarwal SK, Khilnani GC, Kapil A, Dar L, Singh UB, Mirdha BR, et al. Spectrum of pulmonary infections in renal transplant recipients in the tropics: a single center study. Int Urol Nephrol. 2005;37(3):551-559.
doi pubmed - Rana DS, Bhalla AK, Gupta A, Malik M, Gupta A. Enhancing success in the ABO-incompatible kidney transplantation: a case report. Cureus. 2024;16(6):e62350.
doi pubmed - Agarwal S, Maheshwari A, Bajpai M. Large volume plasmapheresis using a single-use immunoadsorption column: A cost-effective approach for desensitization in ABO-incompatible liver transplant. J Clin Apher. 2023;38(5):548-554.
doi pubmed - Ghosh NK, Makki K, Yadav Y, Karan T, Srivastava P, Agarwal A, Vij V. Tailored strategies for emergency ABO-incompatible living donor liver transplantation: a series of three cases. Clin Transplant Res. 2025;39(2):161-168.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Nephrology and Urology is published by Elmer Press Inc.