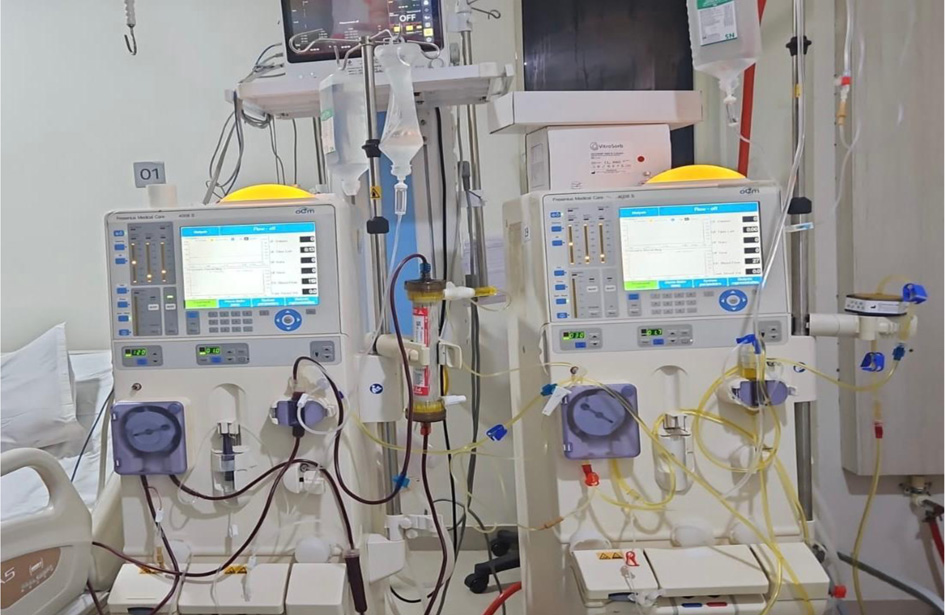
Figure 1. SECORIM® immunoadsorption column setup.
| World Journal of Nephrology and Urology, ISSN 1927-1239 print, 1927-1247 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Nephrol Urol and Elmer Press Inc |
| Journal website https://wjnu.elmerpub.com |
Original Article
Volume 14, Number 2, November 2025, pages 35-43
Successful Usage of Extracorporeal Plasma Perfusion Adsorption Devices Columns for Desensitization Through Immunoadsorption in End-Stage Renal Disease Patients for ABO-Incompatible Kidney Transplant
Figures

Tables
| Step | Description |
|---|---|
| ABOiKT: ABO-incompatible kidney transplantation; HLA: human leukocyte antigen; IA: immunoadsorption. | |
| Donor-recipient mismatch | ABO blood group incompatibility |
| Pre-transplant evaluation | Blood group typing, HLA matching, antibody titers (IgG, IgM) |
| Desensitization initiation | Rituximab infusion, followed by IA or plasmapheresis |
| Surgical procedure | End-to-side vascular anastomosis under general anesthesia |
| Post-operative immunosuppression | Tacrolimus, mycophenolate, steroids, and monitoring of tac/titers/creatinine |
| Patient number | Transplant date | Age (years) | Gender | CKD stage | Donor relationship | Recipient blood group | Donor blood group | Transplant |
|---|---|---|---|---|---|---|---|---|
| CKD: chronic kidney disease. | ||||||||
| 1 | 12/11/2022 | 46 | F | Stage 5 | Husband | B+ | A+ | Successful |
| 2 | 13/01/2023 | 41 | M | Stage 5 | Father | O+ | A+ | Successful |
| 3 | 03/07/2024 | 51 | M | Stage 5 | Wife | O+ | B+ | Successful |
| 4 | 14/03/2024 | 45 | F | Stage 5 | Mother | B+ | A+ | Successful |
| 5 | 1/10/2024 | 42 | F | Stage 5 | Sister | B+ | A+ | Successful |
| 6 | 20/10/2021 | 45 | F | Stage 5 | Mother | A+ | AB+ | Successful |
| 7 | 01/10/2024 | 30 | M | Stage 5 | Wife | O+ | B+ | Successful |
| 8 | 02/05/2024 | 33 | M | Stage 5 | Brother | O+ | A+ | Successful |
| 9 | 12/10/2021 | 41 | M | Stage 5 | Sister | B+ | AB+ | Successful |
| 10 | 21/03/2024 | 32 | M | Stage 5 | Brother | B+ | AB+ | Successful |
| 11 | 16/092022 | 33 | M | Stage 5 | Wife | A+ | B+ | Successful |
| 12 | 29/04/2022 | 28 | M | Stage 5 | Mother | O+ | A+ | Successful |
| 13 | 20/10/2021 | 44 | F | Stage 5 | Mother | A+ | AB+ | Successful |
| 14 | 7/09/2021 | 40 | M | Stage 5 | Mother | B+ | AB+ | Successful |
| 15 | 19/02/2020 | 35 | M | Stage 5 | Maternal uncle | B+ | A+ | Successful |
| 16 | 13/04/2022 | 33 | F | Stage 5 | Father | A+ | AB+ | Successful |
| 17 | 7/02/2022 | 23 | M | Stage 5 | Father | O+ | B+ | Successful |
| 18 | 23/09/2021 | 39 | M | Stage 5 | Wife | O+ | AB+ | Successful |
| 19 | 16/07/2021 | 61 | M | Stage 5 | Wife | O+ | B+ | Successful |
| 20 | 31/01/2020 | 48 | M | Stage 5 | Wife | O+ | B+ | Successful |
| 21 | 13/01/2023 | 44 | M | Stage 5 | Father | O+ | A+ | Successful |
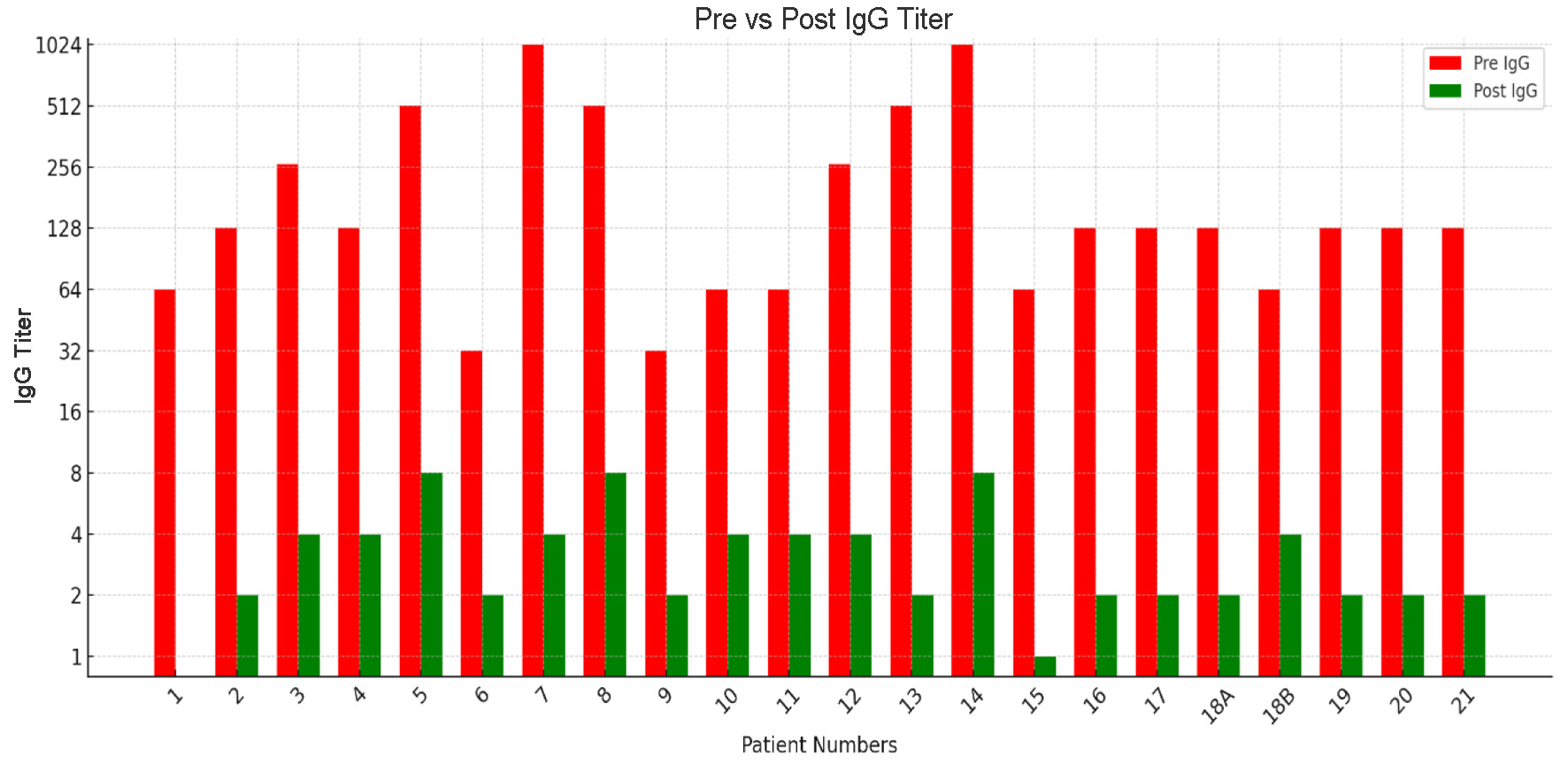
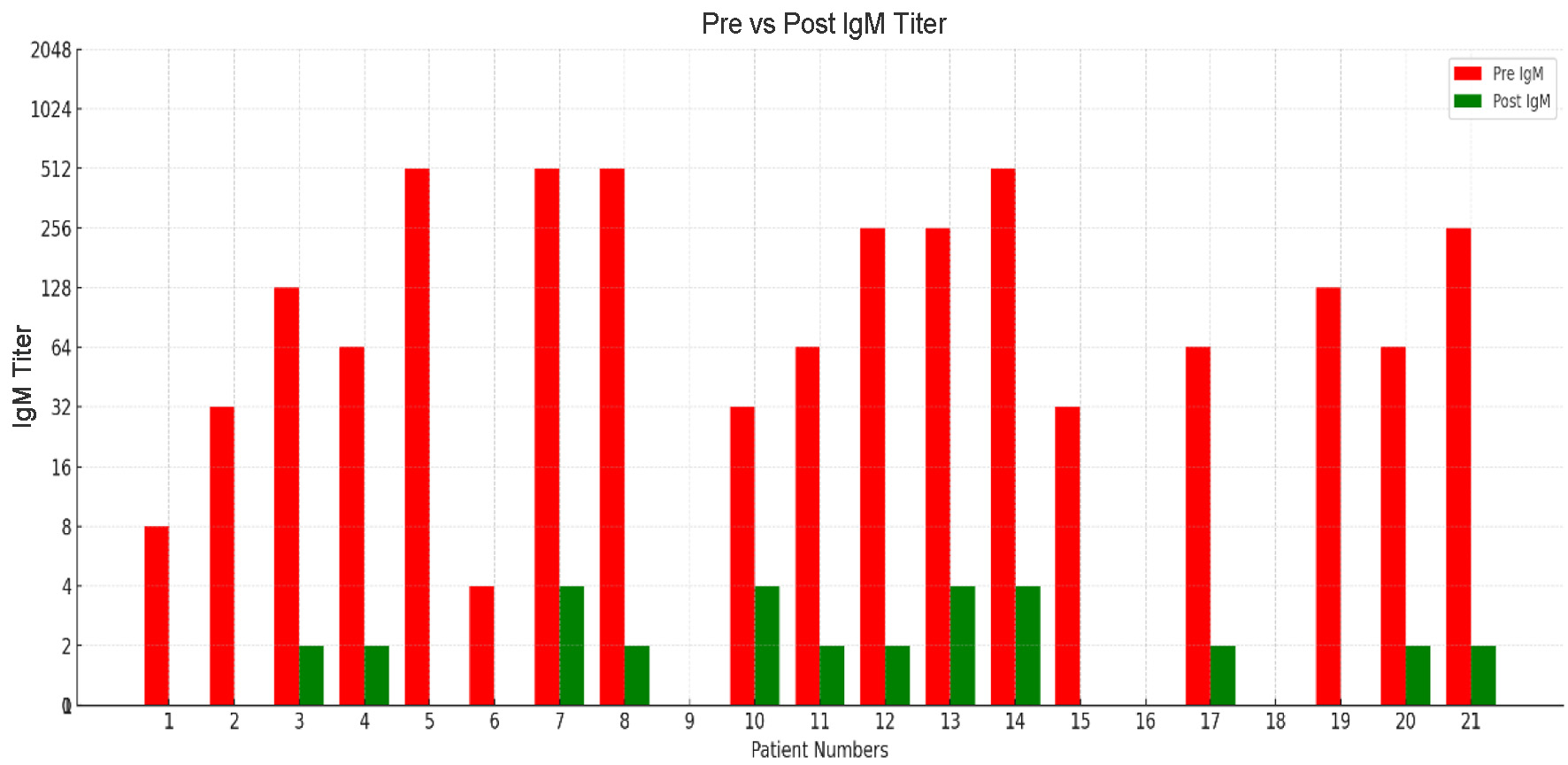
| Patient number | Immunoadsorption sessions | Column used | Pre-IgG titer | Pre-IgM titer | Post-IgG titer | Post-IgM titer |
|---|---|---|---|---|---|---|
| NA: data not available. | ||||||
| 1 | 2 | A | 1:64 | 1:8 | Nil | NA |
| 2 | 2 | A | 1:128 | 1:32 | 1:2 | NA |
| 3 | 2 | B | 1:256 | 1:128 | 1:4 | 1:2 |
| 4 | 1 | A | 1:128 | 1:64 | 1:4 | 1:2 |
| 5 | 2 | A | 1:512 | 1:512 | 1:8 | NA |
| 6 | 1 | B | 1:32 | 1:4 | 1:2 | Nil |
| 7 | 2 | B | 1:1,024 | 1:512 | 1:4 | 1:4 |
| 8 | 2 | A | 1:512 | 1:512 | 1:8 | 1:2 |
| 9 | 1 | A | 1:32 | Nil | 1:2 | NA |
| 10 | 2 | A | 1:64 | 1:32 | 1:4 | 1:4 |
| 11 | 3 | B | 1:64 | 1:64 | 1:4 | 1:2 |
| 12 | 2 | A | 1:256 | 1:256 | 1:4 | 1:2 |
| 13 | 2 | B | 1:512 | 1:256 | 1:2 | 1:4 |
| 14 | 3 | A | 1:1,024 | 1:512 | 1:8 | 1:4 |
| 15 | 1 | A | 1:64 | 1:32 | 1:1 | Nil |
| 16 | 1 | B | 1:128 | NA | 1:2 | NA |
| 17 | 2 | B | 1:128 | 1:64 | 1:2 | 1:2 |
| 18 | 2 | AB | - | - | - | - |
| B | 1:128 | NA | 1:2 | NA | ||
| A | 1:64 | NA | 1:4 | NA | ||
| 19 | 2 | B | 1:128 | 1:128 | 1:2 | 1:1 |
| 20 | 2 | B | 1:128 | 1:64 | 1:2 | 1:2 |
| 21 | 2 | A | 1:128 | 1:256 | 1:2 | 1:2 |
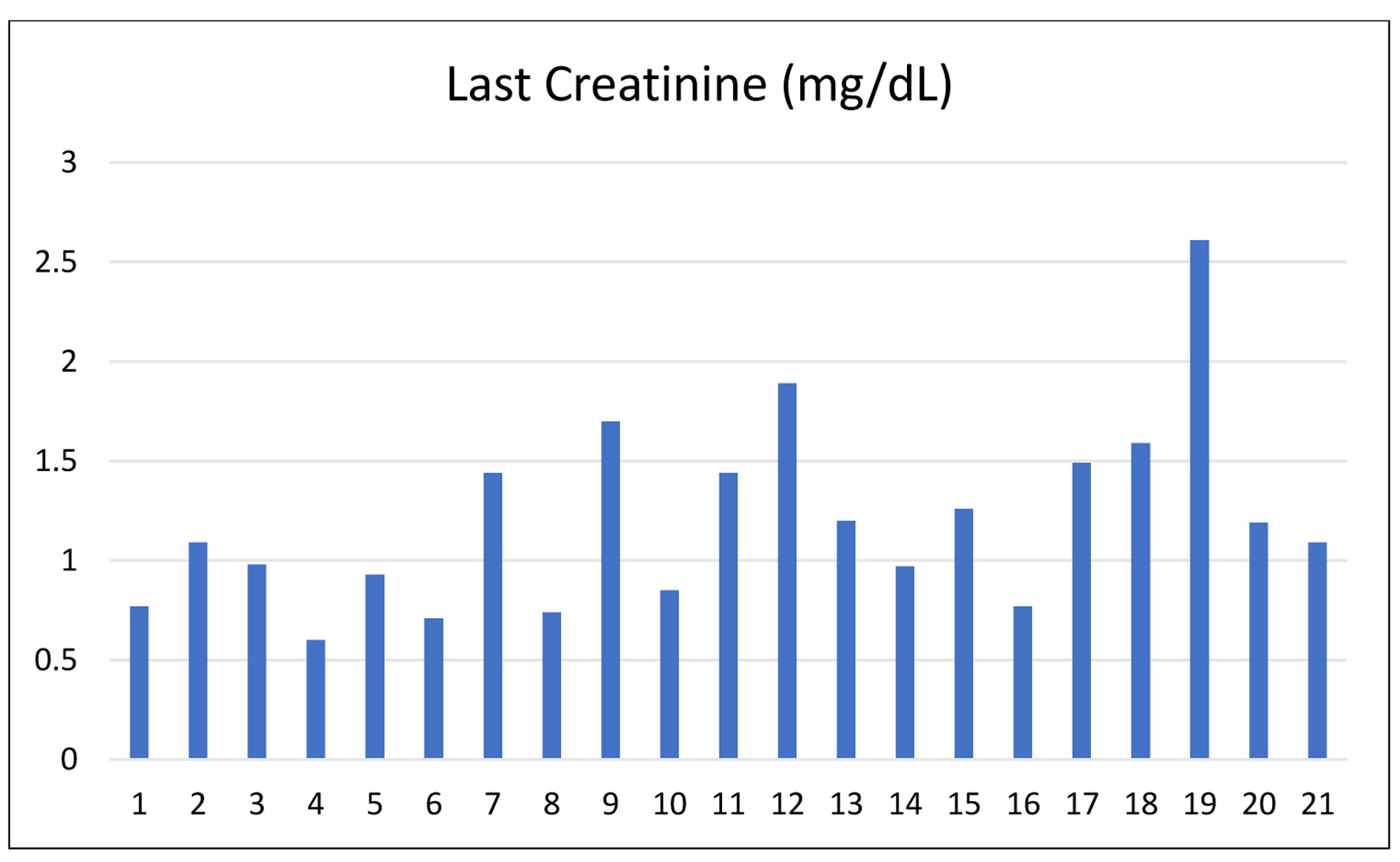
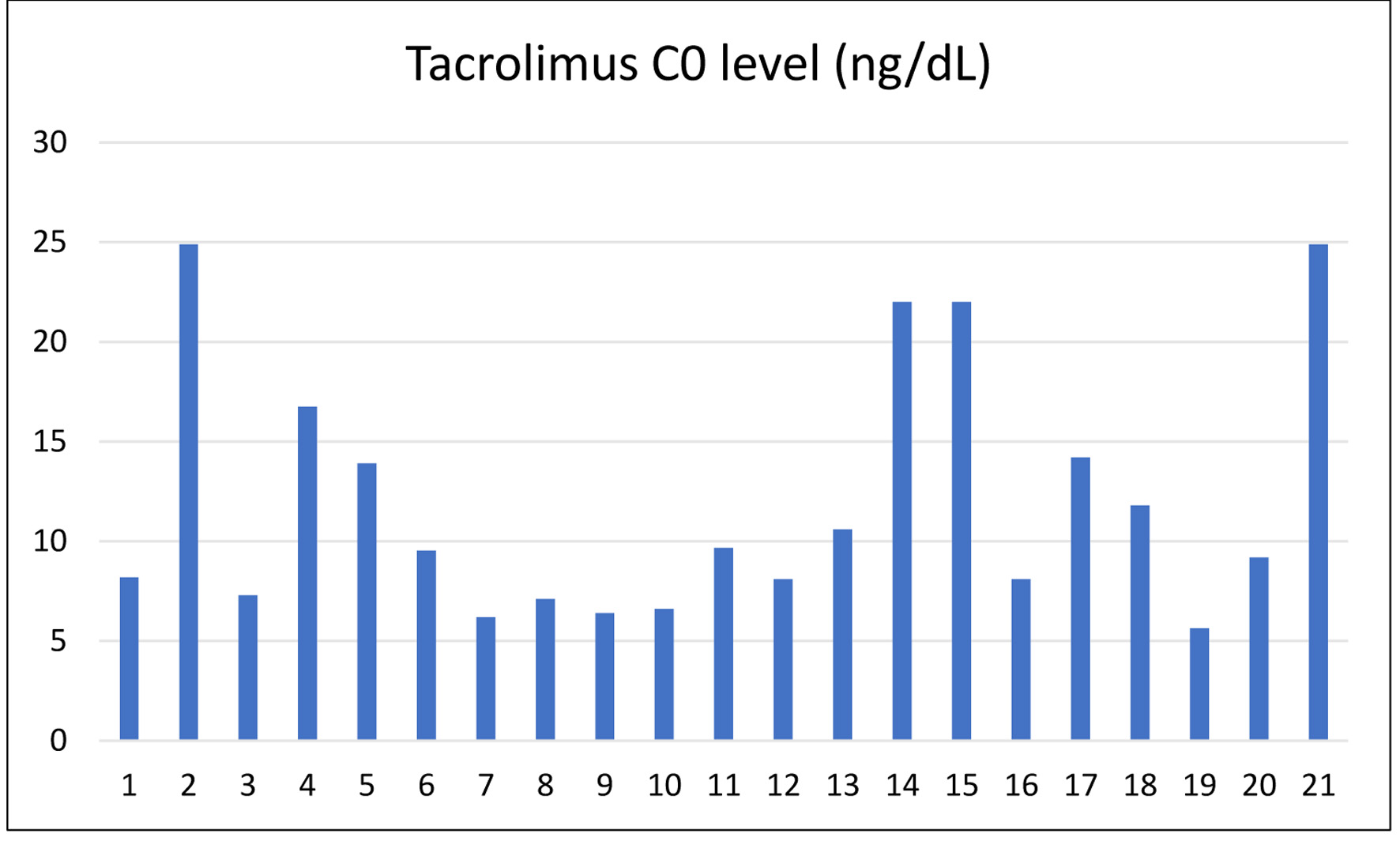
| Patient number | Days from admission to discharge | Last creatinine (mg/dL) | Tacrolimus (ng/mL) |
|---|---|---|---|
| 1 | 12 | 0.77 | 8.2 |
| 2 | 11 | 1.09 | 24.9 |
| 3 | 9 | 0.98 | 7.30 |
| 4 | 9 | 0.6 | 16.75 |
| 5 | 10 | 0.93 | 13.91 |
| 6 | 8 | 0.71 | 9.54 |
| 7 | 10 | 1.44 | 6.19 |
| 8 | 9 | 0.74 | 7.12 |
| 9 | 9 | 1.7 | 6.4 |
| 10 | 9 | 0.85 | 6.6 |
| 11 | 11 | 1.44 | 9.67 |
| 12 | 21 | 1.89 | 8.10 |
| 13 | 6 | 1.2 | 10.6 |
| 14 | 10 | 0.97 | 22 |
| 15 | 9 | 1.26 | 22 |
| 16 | 10 | 0.77 | 8.1 |
| 17 | 9 | 1.49 | 14.2 |
| 18 | 6 | 1.59 | 11.8 |
| 19 | 11 | 2.61 | 5.64 |
| 20 | 10 | 1.19 | 9.2 |
| 21 | 11 | 1.09 | 24.9 |