| World Journal of Nephrology and Urology, ISSN 1927-1239 print, 1927-1247 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Nephrol Urol and Elmer Press Inc |
| Journal website https://wjnu.elmerpub.com |
Review
Volume 14, Number 2, November 2025, pages 27-34
Discordance Between Prostate-Specific Antigen and Positron Emission Tomography Imaging in Recurrent Prostate Cancer
Enoch Chi Ngai Lima , Chi Eung Danforn Lima, b, c, d
aTranslational Research Department, Specialist Medical Services Group, Earlwood, NSW 2206, Australia
bSchool of Life Sciences, University of Technology Sydney, Ultimo, NSW 2007, Australia
cNICM Health Research Institute, Western Sydney University, Westmead, NSW 2145, Australia
dCorresponding Author: Chi Eung Danforn Lim, Translational Research Department, Specialist Medical Services Group, Earlwood NSW 2206, Australia
Manuscript submitted September 9, 2025, accepted October 23, 2025, published online November 8, 2025
Short title: PSA and PET Imaging in Recurrent Prostate Cancer
doi: https://doi.org/10.14740/wjnu1010
- Abstract
- Introduction
- Case Report
- Use of PET Scans in PCa Recurrence Detection
- Diagnostic and Detection Challenges
- Diagnostic Pitfalls and Considerations
- Emerging Imaging Trials
- Conclusion
- References
| Abstract | ▴Top |
Prostate cancer biochemical recurrence (BCR) refers to a rise in prostate-specific antigen (PSA) after treatment. It remains a major diagnostic challenge. Positron emission tomography (PET) imaging, especially with prostate-specific membrane antigen (PSMA) tracers, has emerged as a highly sensitive tool for localizing prostate cancer recurrence. This review examines the use of PET scans in detecting prostate cancer recurrence, evaluates diagnostic challenges and pitfalls, and discusses the limitations of PET in the BCR setting. We also report a case of a 77-year-old man with early BCR after prostatectomy and multimodal therapy, whose PSA climbed progressively from 0.03 to 7.0 ng/mL over 7 years despite salvage treatments. Notably, four consecutive PET scans and a bone scan remained negative for recurrent or metastatic disease, highlighting the limitations of imaging in detecting occult micrometastatic prostate cancer. PET imaging has greatly improved recurrence detection. Still, false negatives and reading pitfalls can affect patient management.
Keywords: Prostate cancer; Biochemical recurrence; Prostate-specific antigen; PET; Prostate-specific membrane antigen
| Introduction | ▴Top |
Prostate cancer (PCa) is one of the most prevalent malignancies in men and a leading cause of cancer mortality [1]. Many men achieve initial control with surgery or radiotherapy. Yet, recurrence marked by rising PSA is common. Approximately 20-30% of men experience biochemical recurrence (BCR) within 10 years after radical prostatectomy, and up to ∼45% after primary radiotherapy [1]. BCR often precedes clinical or radiographic evidence of disease by months or years. Early detection of recurrence is crucial, as timely salvage therapy (e.g., radiotherapy to the prostate bed or systemic therapy) can be potentially curative or disease-prolonging [1]. However, identifying the site of recurrence (local vs. metastatic) is challenging when PSA levels are low and disease burden is minimal. Conventional imaging modalities like computed tomography (CT) and bone scintigraphy have low sensitivity for small or early recurrent lesions [1]. They often fail to detect metastases until the disease volume is relatively large. For instance, bone scans are rarely positive at PSA levels below ∼10 ng/mL and almost never below 0.5 ng/mL [2, 3]. This diagnostic gap can leave clinicians uncertain whether rising PSA reflects residual local disease, nodal metastases, or distant spread.
Molecular imaging with positron emission tomography (PET) has transformed the evaluation of BCR by offering far greater sensitivity for small foci of PCa. PET tracers such as radiolabeled choline and fluciclovine were among the first agents used for PCa recurrence imaging, showing improved accuracy over conventional scans [1]. More recently, PET targeting prostate-specific membrane antigen (PSMA) has demonstrated superior performance in detecting recurrent PCa, even at very low PSA levels [1]. PSMA is a transmembrane protein highly expressed in most prostate adenocarcinomas, providing a molecular target for imaging. PSMA PET/CT can localize tiny metastases in lymph nodes or bones that are occult on CT or bone scan, thereby distinguishing localized from systemic recurrence with much higher confidence [4, 5]. The introduction of PSMA PET represents a paradigm shift; clinical trials have demonstrated that it can detect metastatic lesions in a significant fraction of men with BCR that would otherwise be missed by conventional imaging [3, 5]. Modern guidelines now recommend PSMA PET/CT as the preferred imaging modality for men with BCR after primary therapy [6, 7]. PET results help tailor treatment. They can guide focused radiotherapy or targeted therapy.
Despite these advances, PET imaging is not infallible. There are important limitations and potential pitfalls in interpreting PET scans for PCa recurrence. Very small tumor deposits may evade detection (yielding false-negative scans), especially when PSA is low [8]. Conversely, not every PET-positive lesion represents cancer (false positives can occur from benign conditions) [8]. The case presented below illustrates how recurrent PCa can remain elusive on serial PET scans, despite a clear BCR. Using this case as a backdrop, we discuss the capabilities and limitations of PET in detecting PCa recurrence, compare different imaging modalities (Table 1 [2, 9]), and highlight diagnostic challenges and considerations for clinicians interpreting PET results in the BCR setting.
 Click to view | Table 1. Imaging Modalities for Detecting Prostate Cancer Recurrence |
| Case Report | ▴Top |
The following case illustrates discordance between PSA and imaging findings. A 77-year-old man was diagnosed with high-risk prostate adenocarcinoma (Gleason score 4+5) associated with a PSA of 55 at diagnosis 7 years ago. He underwent radical prostatectomy, which confirmed pT3a disease with a 2 mm extracapsular extension. A pelvic lymph node dissection sampled 19 nodes with no metastases. The patient’s postoperative PSA nadir was 0.03 ng/mL. However, within 1 year, PSA rose to 0.16 ng/mL, consistent with early BCR. He received salvage external beam radiotherapy to the prostate bed and pelvic lymph nodes, along with 6 months of androgen deprivation therapy (ADT). This multimodal salvage treatment brought his PSA back to undetectable levels.
Over the subsequent years, the patient experienced a progressive rise in PSA, indicating recurrent disease despite prior therapy. Documented PSA values were 1.3 ng/mL at approximately 3.5 years post-prostatectomy, 2.1 ng/mL at 4 years, 3.0 ng/mL at 4.5 years, 3.3 ng/mL at 5.4 years, 4.9 ng/mL at 6 years, and 7.0 ng/mL at the beginning of year 7. Between 2021 and 2024, he underwent four PET/CT scans (with radiotracers targeting PCa) and one technetium-99m bone scintigraphy. Despite the steadily rising PSA (from 1.3 to 7 ng/mL over that interval), none of these imaging studies revealed any definite local recurrence or distant metastatic lesions. In other words, all scans were read as negative for active disease, highlighting a discordance between biochemical and radiographic findings. It is important to note that although this patient’s preoperative PSA was markedly elevated at 55 ng/mL, no metastases were found on staging scans or at surgery. This discordance suggests that the tumor cells in this case might have been highly PSA-secreting despite a relatively limited overall tumor burden. PSA production can vary widely between tumors; in some patients, even a small volume of cancer can generate disproportionately high PSA levels, which may explain the absence of detectable metastatic disease despite the high PSA.
In addition to BCR, the patient endured significant treatment-related morbidity. Following prostatectomy and pelvic radiotherapy, he developed chronic urinary urgency and incontinence, as well as bowel dysfunction manifesting as frequency and occasional incontinence. He also has erectile dysfunction. Exposure to the ADT contributed to side effects, including fatigue and decreased libido. These issues are consistent with known adverse effects of PCa therapies and represent important considerations in his long-term care. The ongoing PSA rise despite negative imaging shows the difficulty of managing recurrence when scans show no visible disease.
| Use of PET Scans in PCa Recurrence Detection | ▴Top |
PET imaging is now key in detecting recurrent PCa. It is more sensitive than standard scans. The most widely used PET tracers for recurrent PCa are summarized in Table 1. PSMA-targeted PET tracers (such as 68Ga-PSMA-11 and 18F-DCFPyL) have demonstrated the highest detection rates for recurrent disease. Multiple studies have shown that PSMA PET can identify recurrent lesions even at PSA levels < 0.5 ng/mL in a substantial proportion of patients [8, 9]. For instance, prospective trials report that when PSA levels are between 0.2 and 0.5 ng/mL, PSMA PET scans are positive in approximately 40-60% of cases [8, 9]. Detection rates increase markedly with higher PSA: at PSA levels above 2 ng/mL, PSMA PET can localize disease in over 80-90% of men [1, 8]. This is a dramatic improvement compared to conventional imaging (CT and bone scan), which often detects recurrence in < 20% of men with PSA < 1 ng/mL [2]. In our patient’s case, the use of PSMA PET scans (four times over 3 years) was aimed at finding the source of his rising PSA. PSMA PET is known to outperform other modalities in sensitivity; therefore, the repeatedly negative results were unexpected given his PSA reached 7 ng/mL.
Historically, PET tracers such as 11C-choline or 18F-fluorocholine have been used for BCR imaging. Choline PET/CT offered moderate detection capability but typically required higher PSA levels (> 1 ng/mL) for reasonable sensitivity, and it performed poorly when PSA levels were very low (e.g., < 0.5) [1]. Another FDA-approved tracer, 18F-fluciclovine (Axumin), improved detection of recurrence compared to CT/bone scan and does not rely on prostate-specific targets (it images amino acid uptake). Fluciclovine PET has been useful particularly for localization of pelvic recurrence. However, direct comparisons show that PSMA PET/CT has a significantly higher detection rate than fluciclovine PET at all PSA levels of BCR [6]. For example, one head-to-head study noted that at a PSA level of 0.2 - 0.5 ng/mL, PSMA PET detected recurrence in ∼46% of patients, compared to ∼27% with fluciclovine [6]. The superior sensitivity of PSMA-targeted imaging has made it the new standard. In 2020 - 2021, PSMA PET tracers gained regulatory approvals in many countries for imaging men with suspected recurrence, and professional guidelines (e.g., National Comprehensive Cancer Network and European Association of Urology) endorse PSMA PET/CT for restaging BCR when available [6, 10]. Our patient’s multiple negative PET scans, despite using this advanced technology, highlight that even the best imaging test can fail to detect microscopic disease. Nonetheless, in general practice, a PSMA PET/CT that turns out positive can be invaluable, as it may reveal, for example, a small lymph node metastasis or an isolated bone lesion that is amenable to targeted therapy. This can dramatically change management, such as adding nodal radiation or oligometastatic ablation, or intensifying systemic therapy [5].
Other PET tracers have niche roles. 18F-fluorodeoxyglucose (FDG) PET is not routinely used for typical prostate adenocarcinoma because most tumors are low in glucose metabolism; FDG PET is more helpful in rare aggressive variants (neuroendocrine or highly dedifferentiated disease). Novel PET agents continue to be studied, but PSMA PET remains the most sensitive and specific modality for PCa recurrence currently in clinical use [8]. Importantly, PET scans are often combined with CT (PET/CT) or magnetic resonance imaging (MRI) (PET/MRI) to provide anatomical localization. The CT portion of a PSMA PET/CT allows one to see if a PET-positive spot corresponds to a lymph node, bone, or other structure. In the context of recurrence, PSMA PET/CT can distinguish whether the relapse is likely local (confined to the prostate bed) or distant (lymphatic or hematogenous spread). For example, a positive PSMA PET might show uptake in a pelvic lymph node or a vertebral bone, whereas a negative scan (as in our patient repeatedly) leaves the location indeterminate. Even with these advances, PET scans may still miss small or biologically silent tumors. Table 1 contrasts PET modalities with other imaging techniques in terms of detection performance and limitations in BCR.
| Diagnostic and Detection Challenges | ▴Top |
This patient’s rising PSA but negative imaging shows a key PET limitation. Even advanced PET can miss disease when tumor burden is very low. A negative PSMA PET/CT does not definitively rule out PCa recurrence [8]. Studies have documented that at low PSA values (e.g. < 0.5 ng/mL), at least 45% of men will have no visible lesion on PSMA PET despite biochemical evidence of cancer [10]. In our patient, the PSA had reached notably higher levels (several ng/mL) and it is unusual that repeated PSMA PET scans remained negative. This suggests that his disease was present as occult microscopic deposits below the resolution of imaging - likely small clusters of cancer cells in lymph nodes or bone marrow that were too few or too tiny (≤ 2 - 3 mm) to be detected. PSMA PET’s spatial resolution is on the order of a few millimeters, and very small metastases or disseminated tumor cells will evade detection. Additionally, tumor biology can affect scan sensitivity. While most prostate adenocarcinomas highly express PSMA, there can be heterogeneity; a subset of lesions may have low PSMA expression (for example, dedifferentiated or neuroendocrine-transformed cells), resulting in false-negative PET findings despite tumor presence. Thus, a negative PET does not mean no cancer. It may only mean that the cancer is too small to see. This has important implications: patients with rising PSA should not be falsely reassured by negative scans if the PSA kinetics and clinical risk factors strongly indicate recurrence.
Another diagnostic challenge is determining the timing of imaging. If PET scans are done too early in the course of recurrence (when PSA is very low), they yield negative results that may necessitate repeat scanning later. In this case, multiple serial PETs were performed as the PSA level increased. Generally, the probability of detection rises with PSA level and shorter PSA doubling time [1]. Fast PSA kinetics (short doubling time) are associated with a higher likelihood of PET positivity because aggressive disease tends to produce stronger signals [1]. In our patient, the PSA doubling time accelerated over time (from 0.16 to 7.0 ng/mL in approximately 5 - 6 years), which should have increased the likelihood of PET detecting a lesion. That nothing was found on imaging until very high PSA suggests either an atypical tumor behavior or that the disease remained confined to an anatomical site not easily imaged. One possibility is that his recurrence was largely in microscopic bone metastases without enough reactive change to show on PET or bone scan (since bone scans rely on osteoblastic activity). Another possibility is regional lymph node metastases that were infiltrative but not enlarged; PSMA PET usually catches even sub-centimeter nodal metastases if PSMA-expressing, but it can miss microscopic tumor in normal-sized nodes. Indeed, studies comparing PSMA PET to surgical pathology have found that PET misses a significant fraction of nodal metastases < 5 mm. For example, several studies reported a PSMA PET varying sensitivity of 36.7-84% for detecting positive lymph nodes at the time of salvage lymphadenectomy [11-14]. This means that more than half of small nodal metastases may be missed by imaging. Our patient had prior pelvic lymph node dissection (which was negative) and radiotherapy, so if cancer later seeded pelvic nodes, those could be tiny and in scarred tissue, posing detection difficulties.
An important consideration illustrated by this case is the management of BCR when imaging is negative. Guidelines generally advocate for early salvage therapies based solely on PSA rise, rather than waiting for imaging confirmation of disease [10]. The rationale is that treating when tumor burden is lowest offers the best chance of cure or control. In fact, evidence indicates that men with BCR can benefit from salvage radiotherapy to the prostate bed even if PSMA PET scans show no lesions (presumably because the disease might be microscopic and still located in or near the bed) [1]. In our patient’s history, early salvage radiation was given at PSA 0.15 ng/mL without any imaging-detectable target, a standard approach supported by long-term outcomes. His subsequent rising PSA despite that suggests distant disease outside the treated area. The challenge was that even modern PET imaging could not guide targeted intervention (since no target was seen). In such cases, clinicians may resort to systemic therapy (like ADT) empirically. Long-term ADT causes tumor regression and may lower PSMA expression on cancer cells, especially in hormone-sensitive cancers [15, 16]. PSMA is androgen-regulated; when patients start ADT, PSMA levels may initially paradoxically increase for a couple of weeks, but with longer ADT, the tumor PSMA expression and volume decrease [15, 16]. In practical terms, imaging a patient on ADT can yield false-negative results because the treatment has temporarily suppressed or shrunk metastases. In our patient, the PET scans between 2021 and 2024 were done during a period when he had received ADT for 6 months. If a scan was performed shortly after hormone therapy, it might have missed lesions that could have been visible off therapy. Thus, one diagnostic consideration is the timing of PET relative to ADT - to perform PSMA PET before initiating systemic therapy or after a sufficient washout, to avoid the confounding effects of hormone-induced changes.
Several biological factors can explain negative PSMA PET findings despite BCR. Some PCa cells lose or downregulate PSMA expression, especially after long-term androgen deprivation or dedifferentiation. These low-PSMA clones may grow but remain invisible to PSMA-targeted tracers. In early recurrence, tumor deposits can also be microscopic or dormant, with too few cells to generate a visible PET signal. In addition, some lesions are in areas of low tracer delivery, such as necrotic or poorly vascular tissues. Together, these biological and technical factors contribute to false-negative PSMA PET results even at high PSA levels.
When PSA rises but PSMA PET is negative, clinicians should integrate biochemical data, PSA kinetics, and prior treatment fields. Early salvage therapy can still be justified if recurrence is likely local, even without a visible target. Repeating PET after several months or using an alternative tracer, such as fluciclovine or gastrin-releasing peptide receptor (GRPR)-targeted agents, may reveal disease later. Imaging should ideally be performed before starting or after stopping androgen deprivation to avoid PSMA suppression. As illustrated in Figure 1, a multidisciplinary review helps decide whether to observe, re-image, or initiate systemic therapy.
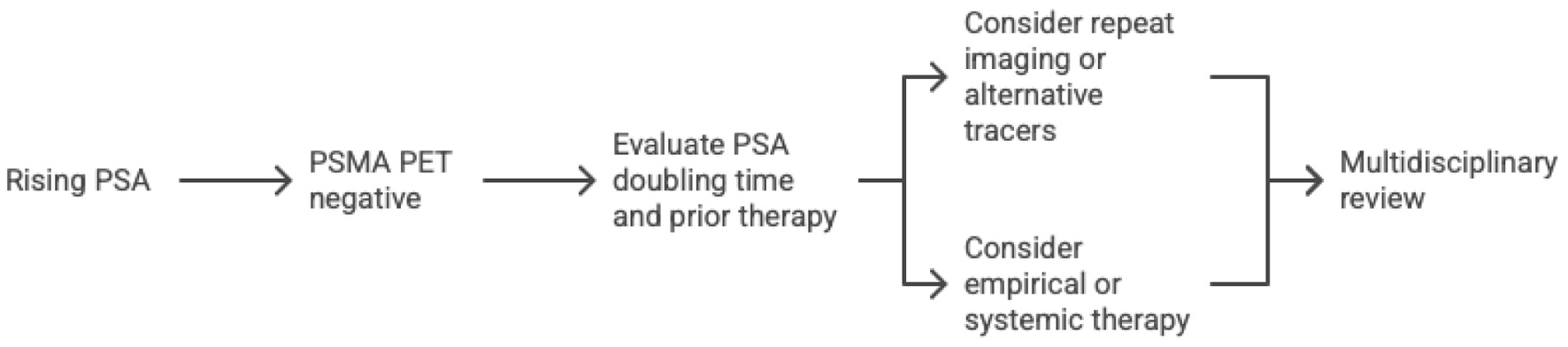 Click for large image | Figure 1. Proposed workflow for managing rising PSA with negative PSMA PET. PET: positron emission tomography; PSA: prostate-specific antigen; PSMA: prostate-specific membrane antigen. |
| Diagnostic Pitfalls and Considerations | ▴Top |
PET scans have improved recurrence detection. Yet, reading them needs caution. One common pitfall is the issue of false-positive findings on PET. PSMA PET, despite high specificity, can produce false positives due to uptake in certain benign or non-prostatic tissues [8]. For example, benign bone abnormalities such as healing fractures, arthritic changes, or fibrous dysplasia can show mild radiotracer uptake. In PSMA PET, an unexplained low-intensity focus in a rib or other bone with no CT correlate often turns out to be benign [8, 16]. Experienced nuclear medicine physicians use standardized uptake values (SUVs) and patterns to differentiate likely metastases from artifact or benign uptake. Typically, true prostate cancer metastases on PSMA PET have intense uptake (SUV significantly above background), whereas false positives (e.g., degenerative bone lesions) have low-grade uptake [8, 17]. The distribution of disease is also a clue; for instance, PCa very rarely metastasizes to superficial inguinal lymph nodes until very late stages [18], so a PSMA-positive inguinal node in an otherwise oligometastatic scenario is more likely a false positive (perhaps from infection or another malignancy) [8]. Other malignancies can express PSMA and cause confusion; isolated cases of PSMA PET highlighting a second cancer (such as an incidental colon or lung tumor) have been reported [19-22]. Therefore, clinicians should correlate PET findings with conventional imaging: if a PET hotspot corresponds to a sclerotic bone lesion with benign features on CT, it should be interpreted with caution. Correlation with MRI can also be useful for equivocal lesions.
When PET does reveal a lesion, another consideration is verification. Because biopsying small metastases is often impractical, PSMA PET-positive findings are usually accepted as true recurrence given the high positive predictive value. However, in ambiguous cases, confirmation may be pursued. For example, if a single suspicious lymph node lights up, one might perform a targeted biopsy or surgically remove the node to obtain pathology. In the context of BCR, however, positive PET findings are frequently used to direct therapy (e.g., stereotactic radiation to a metastatic node) without biopsy, as the tracer’s specificity is high. The larger problem is the presence of ambiguous or borderline findings. To overcome this, one may observe and repeat the scan after a short interval to see if the signal progresses (true metastases tend to grow/increase uptake, whereas benign uptake stays stable) [8]. Another approach mentioned earlier is to leverage therapy as a diagnostic test; for instance, start ADT and then re-scan in a couple of months to see if a dubious PET signal disappears (if it does, it was likely cancer) [8]. These strategies acknowledge that PET interpretation is not black-and-white and sometimes requires clinical context and follow-up.
The case also brings forth a broader consideration: patient management should not rely solely on imaging. Negative imaging should be weighed alongside PSA trends, histopathology, and clinical risk factors. The consistent rise in PSA strongly indicated malignancy, despite the negative scans [23]. The care team needed to manage a “PSA-only” recurrence. Options in such instances include continuing ADT or enrolling in clinical trials for novel systemic therapies, since no localized target is identified. Moreover, the patient’s quality of life issues (urinary/bowel symptoms and hormonal side effects) must be balanced with any aggressive intervention. Imaging aims to improve outcomes, not just show disease. If PSA is high but scans are negative, treatment can still proceed. Conversely, when imaging is positive, it must be ensured that treating what is seen will truly benefit the patient. For example, spotting one bone metastasis on PET is valuable, but one must consider that other microscopic disease might exist beyond the visible one, guiding the decision to add systemic therapy even if oligometastatic treatment is done.
In summary, PET scans, particularly PSMA PET/CT, have revolutionized the detection of recurrent PCa by identifying disease at PSA levels where conventional scans fail. The availability of the scans enables tailored salvage treatments by pinpointing metastases. Yet, physicians must remain aware of their limitations. False negatives can occur at low tumor volume or due to tumor biology [23], and false positives require careful discrimination. Integrating PET results with the clinical picture and not hesitating to treat empirically (or to verify findings) when appropriate is essential. With ongoing advancements in imaging and theranostics, these challenges are the focus of active research, but at present the mantra is: use PET to guide management, but do not let it solely dictate management in contradiction to clinical evidence.
| Emerging Imaging Trials | ▴Top |
Approximately 20% of men with BCR have negative PSMA PET scans [24-27]. For these patients, new PET tracers targeting GRPR (a “bombesin” receptor) are being tested [28]. GRPR is highly overexpressed in PCa - Markwalder and Reubi found GRPRs present in all examined invasive prostate adenocarcinomas [29], so bombesin analogs can image disease even when PSMA is low. In Australia, the phase II “BOP” trial (NCT05376447) is now recruiting men with BCR and negative or low-PSMA scans to receive 64Cu-SAR-Bombesin PET [28]. This agent binds GRPR, which occurs on up to ∼100% of prostate tumors [29-33].
Preliminary data support this approach. In a pilot series at St Vincent’s Hospital, Australia, four men with rising PSA and negative conventional imaging were imaged with 64Cu-SAR-Bombesin PET, and it detected lesions in all four, leading to changes in their management [34]. Likewise, Baratto et al reported that GRPR-directed 68Ga-RM2 PET identified recurrence in a few patients that PSMA PET missed: RM2 PET was positive in 35/50 BCR patients versus 37/50 on PSMA PET, and the two scans together detected lesions not seen by the other [26]. In that series, RM2 PET found seven lesions in four patients that PSMA PET had missed [13]. More recently, Duan et al showed that 68Ga-NeoB PET (another GRPR ligand) detected 14 lesions in six of 27 men that were false-negative on a PSMA tracer [35]. Together these studies illustrate that GRPR-targeted imaging can reveal occult disease in a subset of PSMA-negative patients. Table 2 illustrates the common PET tracers for recurrent PCa.
 Click to view | Table 2. Common PET Tracers for Recurrent Prostate Cancer |
| Conclusion | ▴Top |
PET imaging has become a central tool for evaluating PCa recurrence. It offers much higher sensitivity than traditional scans but still has important limitations. Microscopic or PSMA-low disease may remain invisible, so results must always be interpreted in clinical context. When PSA rises but PET is negative, management should rely on PSA trends, pathology, and patient risk factors rather than imaging alone. New tracers targeting GRPR and other receptors may soon help detect lesions missed by PSMA PET. Key clinical takeaways are that PET scans greatly improve recurrence detection, but a negative scan does not exclude disease. Clinical judgment and multidisciplinary input remain essential in guiding management.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None of the authors declared financial and non-financial relationships, activities, or any conflict of interest regarding this manuscript.
Informed Consent
Written informed consent for publication of the case presentation was obtained from the patient.
Author Contributions
ENCL: conceptualization, data curation, methodology, formal analysis, investigation, project administration, resources, software, writing – original draft, writing – review and editing. CEDL: conceptualization resources, visualization, software, funding, validation, supervision, writing – review and editing.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
AI Use Declaration
The authors declare that no generative artificial intelligence (AI) or AI-assisted technologies were used in the writing, editing, or preparation of this manuscript.
| References | ▴Top |
- Haidar M, Abi-Ghanem AS, Moukaddam H, Jebai ME, Al Zakleet S, Al Rayess S, Akkawi AR, et al. (68)Ga-PSMA PET/CT in early relapsed prostate cancer patients after radical therapy. Sci Rep. 2022;12(1):20500.
doi pubmed - Wang L, Wang L, Wang X, Wu D. The Evolving Role of PSMA-PET/CT in prostate cancer management: an umbrella review of diagnostic restaging, therapeutic redirection, and survival impact. Curr Oncol Rep. 2025;27(6):774-787.
doi pubmed - Klaassen Z. PSMA and beyond 2025: can we kill the bone scan? UroToday. 2025. Available from: https://www.urotoday.com/conference-highlights/2025-ucsf-ucla-psma-conference/159329-psma-and-beyond-2025-can-we-kill-the-bone-scan.html.
- Fortuin A, Rooij M, Zamecnik P, Haberkorn U, Barentsz J. Molecular and functional imaging for detection of lymph node metastases in prostate cancer. Int J Mol Sci. 2013;14(7):13842-13875.
doi pubmed - Philips C. PSMA PET-CT accurately detects prostate cancer spread, trial shows. Natl Cancer Inst. 2020. Available from: https://www.cancer.gov/news-events/cancer-currents-blog/2020/prostate-cancer-psma-pet-ct-metastasis.
- Sayyid R, Klaassen Z. The current landscape of PSMA PET imaging in prostate cancer: evaluating men with biochemical recurrence. UroToday. 2022. Available from: https://www.urotoday.com/library-resources/imaging-center/139875-the-current-landscape-of-psma-pet-imaging-in-prostate-cancer-evaluating-men-with-biochemical-recurrence.html.
- Tilki D, van den Bergh RCN, Briers E, Van den Broeck T, Brunckhorst O, Darraugh J, Eberli D, et al. EAU-EANM-ESTRO-ESUR-ISUP-SIOG guidelines on prostate cancer. Part II-2024 update: treatment of relapsing and metastatic prostate cancer. Eur Urol. 2024;86(2):164-182.
doi pubmed - Urology Times. PSMA-PET accuracy, false negative/positive rates. Urology Times J. 2024;52(04). Available from: https://www.urologytimes.com/view/dr-reiter-discusses-accuracy-of-psma-pet-scans.
- Wang R, Shen G, Huang M, Tian R. The Diagnostic Role of (18)F-Choline, (18)F-Fluciclovine and (18)F-PSMA PET/CT in the detection of prostate cancer with biochemical recurrence: a meta-analysis. Front Oncol. 2021;11:684629.
doi pubmed - Kranzbuhler B, Muller J, Becker AS, Garcia Schuler HI, Muehlematter U, Fankhauser CD, Kedzia S, et al. Detection rate and localization of prostate cancer recurrence using (68)Ga-PSMA-11 PET/MRI in patients with low PSA Values </= 0.5 ng/mL. J Nucl Med. 2020;61(2):194-201.
doi pubmed - Dundee P, Gross T, Moran D, Ryan A, Ballok Z, Peters J, et al. Ga-PSMA PET: still just the tip of the iceberg. Urology. 2018;120:123-139.
doi - Kimura S, Abufaraj M, Janisch F, Iwata T, Parizi MK, Foerster B, Fossati N, et al. Performance of [(68)Ga] Ga-PSMA 11 PET for detecting prostate cancer in the lymph nodes before salvage lymph node dissection: a systematic review and meta-analysis. Prostate Cancer Prostatic Dis. 2020;23(1):1-10.
doi pubmed - Rauscher I, Maurer T, Beer AJ, Graner FP, Haller B, Weirich G, Doherty A, et al. Value of 68Ga-PSMA HBED-CC PET for the assessment of lymph node metastases in prostate cancer patients with biochemical recurrence: comparison with histopathology after salvage lymphadenectomy. J Nucl Med. 2016;57(11):1713-1719.
doi pubmed - Sprute K, Kramer V, Koerber SA, Meneses M, Fernandez R, Soza-Ried C, Eiber M, et al. Diagnostic accuracy of (18)F-PSMA-1007 PET/CT imaging for lymph node staging of prostate carcinoma in primary and biochemical recurrence. J Nucl Med. 2021;62(2):208-213.
doi pubmed - Afshar-Oromieh A, Debus N, Uhrig M, Hope TA, Evans MJ, Holland-Letz T, Giesel FL, et al. Impact of long-term androgen deprivation therapy on PSMA ligand PET/CT in patients with castration-sensitive prostate cancer. Eur J Nucl Med Mol Imaging. 2018;45(12):2045-2054.
doi pubmed - Hoberuck S, Lock S, Winzer R, Zophel K, Froehner M, Fedders D, Kotzerke J, et al. [(68)Ga]Ga-PSMA-11 PET before and after initial long-term androgen deprivation in patients with newly diagnosed prostate cancer: a retrospective single-center study. EJNMMI Res. 2020;10(1):135.
doi pubmed - Guo J, Chen C, Xiong L, Xiao L, Wei Y, Sun C. Analysis of the application value of SUV in quantitative SPECT/CT for diagnosing benign and malignant bone lesions. Hell J Nucl Med. 2024;27(3):198-205.
doi pubmed - Woo S, Becker AS, Ghafoor S, Barbosa FG, Arita Y, Vargas HA. Inguinal lymph node metastases from prostate cancer: clinical, pathology, and multimodality imaging considerations. Radiol Bras. 2024;57:e20240013.
doi pubmed - Florou VA, Reyes DK, Pienta KJ. Incidental discovery of gastrointestinal stromal tumor via PSMA-PET/CT imaging: Insights from a case report. Urol Case Rep. 2025;58:102926.
doi pubmed - Sharma P. 68Ga-PSMA-Avid small cell lung cancer on PET/CT: incidental second malignancy in treated prostate cancer. Clin Nucl Med. 2020;45(12):1016-1017.
doi pubmed - Gupta M, Choudhury PS, Gupta G, Gandhi J. Metastasis in urothelial carcinoma mimicking prostate cancer metastasis in Ga-68 prostate-specific membrane antigen positron emission tomography-computed tomography in a case of synchronous malignancy. Indian J Nucl Med. 2016;31(3):222-224.
doi pubmed - Anconina R, Hod N, Levin D, Ezroh Kazap D, Lantsberg S. Incidental detection of metastatic malignant melanoma on 68ga-prostate-specific membrane antigen PET/CT imaging: correlative imaging with FDG PET/CT and review of the literature. Clin Nucl Med. 2018;43(3):204-206.
doi pubmed - Adebahr S, Althaus A, Scharl S, Strouthos I, Farolfi A, Serani F, Lanzafame H, et al. The prognostic significance of a negative PSMA-PET scan prior to salvage radiotherapy following radical prostatectomy. Eur J Nucl Med Mol Imaging. 2024;51(2):558-567.
doi pubmed - Afshar-Oromieh A, Holland-Letz T, Giesel FL, Kratochwil C, Mier W, Haufe S, Debus N, et al. Diagnostic performance of (68)Ga-PSMA-11 (HBED-CC) PET/CT in patients with recurrent prostate cancer: evaluation in 1007 patients. Eur J Nucl Med Mol Imaging. 2017;44(8):1258-1268.
doi pubmed - Ferraro DA, Ruschoff JH, Muehlematter UJ, Kranzbuhler B, Muller J, Messerli M, Husmann L, et al. Immunohistochemical PSMA expression patterns of primary prostate cancer tissue are associated with the detection rate of biochemical recurrence with (68)Ga-PSMA-11-PET. Theranostics. 2020;10(14):6082-6094.
doi pubmed - Baratto L, Song H, Duan H, Hatami N, Bagshaw HP, Buyyounouski M, Hancock S, et al. PSMA- and GRPR-targeted PET: results from 50 patients with biochemically recurrent prostate cancer. J Nucl Med. 2021;62(11):1545-1549.
doi pubmed - Mapelli P, Ghezzo S, Samanes Gajate AM, Preza E, Palmisano A, Cucchiara V, Brembilla G, et al. (68)Ga-PSMA and (68)Ga-DOTA-RM2 PET/MRI in recurrent prostate cancer: diagnostic performance and association with clinical and histopathological data. Cancers (Basel). 2022;14(2):334.
doi pubmed - Clarity Pharmaceuticals. Imaging trial with SAR-Bombesin in prostate cancer opens for recruitment in Australia. Clarity Pharmaceuticals. 2024. Available from: https://www.claritypharmaceuticals.com/news/bop_open/.
- Markwalder R, Reubi JC. Gastrin-releasing peptide receptors in the human prostate: relation to neoplastic transformation. Cancer Res. 1999;59(5):1152-1159.
pubmed - Fleischmann A, Waser B, Reubi JC. High expression of gastrin-releasing peptide receptors in the vascular bed of urinary tract cancers: promising candidates for vascular targeting applications. Endocr Relat Cancer. 2009;16(2):623-633.
doi pubmed - Ananias HJ, van den Heuvel MC, Helfrich W, de Jong IJ. Expression of the gastrin-releasing peptide receptor, the prostate stem cell antigen and the prostate-specific membrane antigen in lymph node and bone metastases of prostate cancer. Prostate. 2009;69(10):1101-1108.
doi pubmed - Reubi JC, Wenger S, Schmuckli-Maurer J, Schaer JC, Gugger M. Bombesin receptor subtypes in human cancers: detection with the universal radioligand (125)I-[D-TYR(6), beta-ALA(11), PHE(13), NLE(14)] bombesin(6-14). Clin Cancer Res. 2002;8(4):1139-1146.
pubmed - Sun B, Halmos G, Schally AV, Wang X, Martinez M. Presence of receptors for bombesin/gastrin-releasing peptide and mRNA for three receptor subtypes in human prostate cancers. Prostate. 2000;42(4):295-303.
doi pubmed - Niketh J, Bao H, Emmett L. 64Cu-SAR-Bombesin PET-CT for the detection of biochemically recurrent PSMA-PET negative prostate cancer: a case series. Asia Pac J Clin Oncol. 2022;18(Suppl 1):55.
doi - Duan H, Song H, Davidzon GA, Moradi F, Liang T, Loening A, Vasanawala S, et al. Prospective comparison of (68)Ga-NeoB and (68)Ga-PSMA-R2 PET/MRI in patients with biochemically recurrent prostate cancer. J Nucl Med. 2024;65(6):897-903.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Nephrology and Urology is published by Elmer Press Inc.