| World Journal of Nephrology and Urology, ISSN 1927-1239 print, 1927-1247 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Nephrol Urol and Elmer Press Inc |
| Journal website https://wjnu.elmerpub.com |
Review
Volume 000, Number 000, May 2025, pages 000-000
Are Your Kidneys Ok? Detect Early to Protect Kidney Health
Joseph A. Vassalottia, b, n, Anna Francisc, n, Augusto Cesar Soares Dos Santos Jrd, Ricardo Correa-Rottere, Dina Abdellatiff, Li-Li Hsiaog, Stefanos Roumeliotish, o, Agnes Harisi, Latha A. Kumaraswamij, Siu-Fai Luik, Alessandro Balduccil, Vassilios Liakopoulosh, World Kidney Day Joint Steering Committeem
aDepartment of Medicine-Renal Medicine, Mount Sinai Hospital, New York, New York, USA
bNational Kidney Foundation, Inc., New York, NY, USA
cDepartment of Nephrology, Queensland Children’s Hospital, South Brisbane, Queensland, Australia
dFaculdade Ciencias Medicas de Minas Gerais, Brazil, Hospital das Clinicas, Ebserh, Universidade Federal de Minas Gerais, Brazil
eInstituto Nacional de Ciencias Medicas y Nutricion Salvador Zubiran, Mexico City, Mexico
fDepartment of Nephrology, Cairo University Hospital, Cairo, Egypt
gRenal Division, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, USA
hSecond Department of Nephrology, AHEPA University Hospital Medical School, Aristotle University of Thessaloniki, Thessaloniki, Greece
iNephrology Department, Peterfy Hospital, Budapest, Hungary
jTamilnad Kidney Research (TANKER) Foundation, Chennai, India
kDivision of Health System, Policy and Management, Jockey Club School of Public Health and Primary Care, The Chinese University of Hong Kong, Hong Kong, China
lItalian Kidney Foundation, Rome, Italy
mThe World Kidney Day Joint Steering Committee includes Valerie A. Luyckx, Marcello Tonelli, Ifeoma Ulasi, Vivekanand Jha, Marina Wainstein, Siddiq Anwar, Daniel O’Hara, Elliot K. Tannor, Jorge Cerda, Elena Cervantes, and Maria Carlota Gonzalez.
nThese authors shared the first authorship.
oCorresponding Author: Stefanos Roumeliotis, Second Department of Nephrology, AHEPA University Hospital Medical School, Aristotle University of Thessaloniki, Thessaloniki, Greece
Manuscript submitted April 18, 2025, accepted April 25, 2025, published online May 4, 2025
Short title: Are Your Kidneys Ok?
doi: https://doi.org/10.14740/wjnu1005
- Abstract
- Introduction
- Epidemiology and Complications of Kidney Disease
- Who Is at Risk of Kidney Disease?
- How Can We Check Kidney Health?
- Are Your Kidneys Okay?
- Potential Benefits of Early Detection
- Challenges and Solutions for Implementation
- Conclusion: A Call to Action
- References
| Abstract | ▴Top |
Early identification of kidney disease can protect kidney health, prevent kidney disease progression and related complications, reduce cardiovascular disease risk and decrease mortality. We must ask “Are your kidneys ok?” using serum creatinine to estimate kidney function and urine albumin to assess for kidney and endothelial damage. Evaluation for causes and risk factors for chronic kidney disease (CKD) includes testing for diabetes and measurement of blood pressure and body mass index. This World Kidney Day we assert that case-finding in high-risk populations, or even population level screening, can decrease the burden of kidney disease globally. Early-stage CKD is asymptomatic, simple to test for and recent paradigm shifting CKD treatments such as sodium glucose co-transporter-2 inhibitors dramatically improve outcomes and favor the cost-benefit analysis for screening or case-finding programs. Despite this, numerous barriers exist, including resource allocation, healthcare funding, healthcare infrastructure and healthcare-professional and population awareness of kidney disease. Coordinated efforts by major kidney non-governmental organizations to prioritize the kidney health agenda for governments and aligning early detection efforts with other current programs will maximize efficiencies.
Keywords: Screening; Case finding; Chronic kidney disease; Albuminuria; Proteinuria; Prevention
| Introduction | ▴Top |
Timely treatment is the primary strategy to protect kidney health, prevent kidney disease progression and related complications, reduce cardiovascular disease risk and prevent premature kidney-related and cardiovascular mortality [1-3]. International population assessments show low awareness and low detection of kidney disease and substantial gaps in treatment [2]. People with kidney failure universally express the preference for having been diagnosed early in their disease trajectory to allow more time for educational, lifestyle and pharmacologic interventions [4]. Therefore, increasing knowledge and implementing sustainable solutions for early detection of kidney disease to protect kidney health are public health priorities [2, 3].
| Epidemiology and Complications of Kidney Disease | ▴Top |
Chronic kidney disease (CKD) is prevalent, affecting 10% of the world’s population, or over 700 million people [5]. Almost 80% of the population with CKD reside in low-income countries (LICs) and lower middle-income countries (LMICs), with approximately 1/3 of the known affected population living in China and India alone [5, 6]. Prevalence of CKD increased by 33% between 1990 and 2017 [5]. Increasing prevalence of CKD is driven by population growth, aging and the obesity epidemic, resulting in higher prevalence of two major risk factors for CKD: type-2 diabetes (T2DM) and hypertension. In addition, risk factors for CKD beyond cardiometabolic conditions contribute to the rising burden of kidney disease, including social deprivation, pregnancy-related acute kidney injury (AKI), preterm birth and increasing environmental threats (infections, toxins, climate change, air pollution) [5, 7]. These threats disproportionately affect people in LICs and LMICs [8].
Undetected and untreated CKD is more likely to progress to kidney failure and cause premature morbidity and mortality. Globally, more people died in 2019 of cardiovascular disease (CVD) attributed to reduced kidney function (1.7 million people) than kidney disease alone (1.4 million) [5]. CKD is expected to rise to the 5th most common cause of years of life lost by 2040, surpassing type 2 diabetes, Alzheimer’s disease and road injuries [9]. The rising mortality of kidney disease is remarkable in contrast to other non-communicable diseases (NCDs) such as CVD, stroke and respiratory disease, which are projected to experience a decline in mortality [8]. Even in early stage CKD, multi-system morbidity decreases quality of life. In particular, mild cognitive impairment is associated with early stage CKD and it is possible that early CKD detection and treatment could slow cognitive decline and reduce the risk of dementia [10]. CKD in children has profound additional effects, threatening growth and cognitive development and with lifelong health and quality of life implications [11, 12]. The number of people on kidney failure replacement therapy (KFRT) - dialysis and transplantation - is anticipated to more than double to 5.4 million from 2010 to 2030 [13, 14]. KFRT, especially hemodialysis, is unavailable or unaffordable to many in LICs and LMICs, contributing to millions of deaths annually. LICs and LMICs comprise 48% of the global population but account for only 7% of the treated kidney failure population [15].
| Who Is at Risk of Kidney Disease? | ▴Top |
Testing people at high-risk for kidney disease (case-finding) limits potential harms and false-positive test results compared with general population screening that should only be considered in high-income countries (HICs). Limiting testing to those at increased risk of CKD would still capture a large proportion of the global population. Moreover, targeted case-finding in patients at high risk of CKD, is not optimally performed even in HICs. About 1 in 3 people worldwide have diabetes and/or hypertension. There is a bidirectional relationship between cardiovascular disease and CKD, with each increasing the risk of the other. The American Heart Association and European Society of Cardiology call for testing those with cardiovascular disease for CKD, as part of routine cardiovascular assessments [1, 16].
Other CKD risk factors include family history of kidney disease (e.g. APOL1-mediated kidney disease common in people of West African ancestry), prior AKI, pregnancy-related kidney disease (e.g. pre-eclampsia), malignancy, autoimmune disorders (systemic lupus erythematosus, vasculitis), individuals born with low birth weight or pre-term, obstructive uropathy, recurrent kidney stones, and congenital anomalies of the kidney and urinary tract (CAKUT) (Fig. 1) [3]. The social determinants of health strongly affect CKD risk, both for individuals and at a country level. In LICs and LMICs, heat stress for agricultural workers is thought to cause CKD of unknown etiology, an increasingly recognized major global cause of CKD [17]. In addition, envenomations, environmental toxins, traditional medicines and infections (viral hepatitis B or C, HIV, and parasites) deserve consideration as risk groups, especially in endemic areas [18, 19].
 Click for large image | Figure 1. Risk factors for chronic kidney disease (CKD). |
| How Can We Check Kidney Health? | ▴Top |
Conceptually, there are three levels of CKD prevention. Primary prevention reduces the incidence of CKD by treating risk factors; secondary prevention reduces progression and complications in those with detected CKD; and, tertiary prevention improves outcomes in those with kidney failure by improving management, such as improved vaccination or optimal dialysis delivery [20]. Primary and secondary prevention strategies can utilize the eight golden rules for kidney health promotion; healthy diet, adequate hydration, physical activity, blood pressure monitoring and control, glycemic monitoring and control, avoidance of nicotine, avoidance of regular use of non-steroidal anti-inflammatory drugs and targeted testing for those with risk factors [21]. Five of these are identical to “Life’s Essential 8” rules for improving and maintaining cardiovascular health which also include healthy weight, adequate sleep and lipid management [22]. Early detection focuses on secondary CKD prevention that involves protecting kidney health and reducing cardiovascular risk.
| Are Your Kidneys Okay? | ▴Top |
Globally, early detection of CKD is rare, haphazard and even less likely to occur in LICs or LMICs. Currently, only three countries have a national program to actively test for CKD in at-risk populations and a further 17 countries actively test at-risk population during routine health encounters [23]. Even in HICs, albuminuria is not assessed in over half of people with T2DM and/or hypertension [24-26]. Startlingly, in those with documented reduced kidney function, a diagnosis of CKD is often missing. A study in HICs showed absence of CKD diagnosis among 62-96% of the population with laboratory evidence of CKD stage G3 [27].
We recommend that healthcare professionals perform the following tests for all risk groups to assess kidney health (Fig. 2):
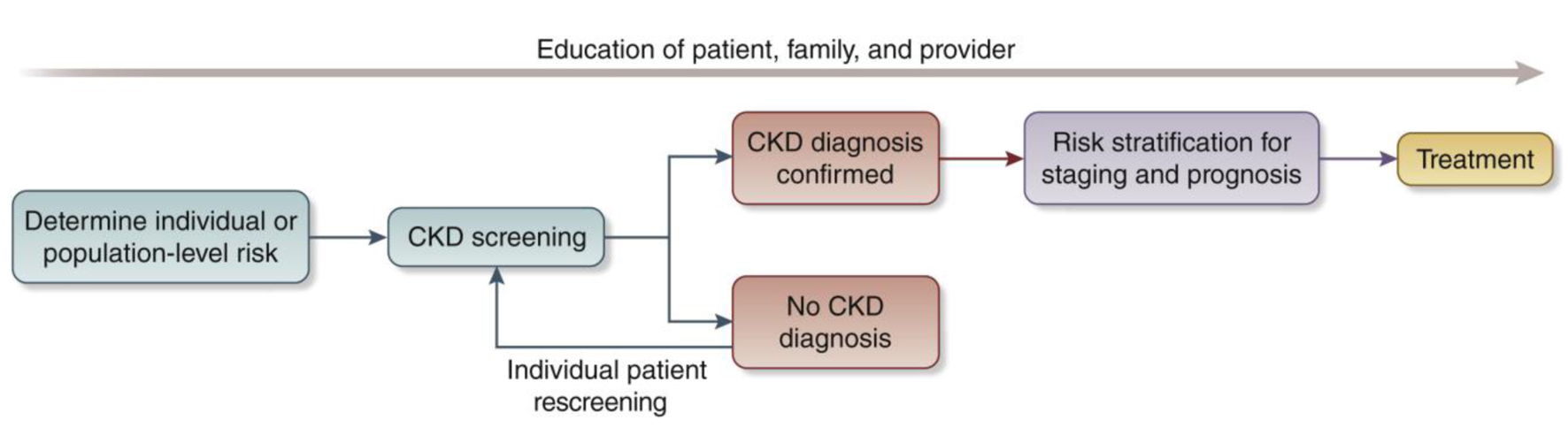 Click for large image | Figure 2. Conceptual framework of a chronic kidney disease (CKD) testing, risk stratification, and treatment program [30]. |
- Blood pressure measurements as hypertension is the most prevalent risk factor for kidney disease worldwide [3, 28, 29].
- Body mass index (BMI) since obesity is epidemiologically associated with CKD risk indirectly through T2DM and hypertension and as an independent risk factor. Visceral adiposity contributes to monocyte microinflammation and cardiometabolic kidney risk [3, 28, 29].
- Testing for diabetes with glycosylated hemoglobin or fasting blood sugar or random glucose is part of kidney health assessment as T2DM is a common risk factor [3, 28, 29].
- Evaluating kidney function by using serum creatinine to estimate GFR (eGFR), is recommended in all settings [3]. GFR should be estimated with a validated, race-free equation appropriate for the country or region and age group [3]. In general, the eGFR < 60 mL/min/1.73 m2 is the threshold for CKD in adults and children, although a threshold of < 90 mL/min/1.73 m2 can be flagged as “low” in children and adolescents over the age of 2 years [3]. A limitation of creatinine-based eGFR is that creatinine is also a marker of nutrition and muscle mass. Therefore, states of malnutrition and frailty overestimate kidney function [3, 30]. Thus, eGFR using the combination of serum creatinine and cystatin C is generally more accurate than either biomarker alone in most clinical contexts. However, the feasibility of cystatin C use is mainly limited to HICs because of assay availability and cost relative to creatinine [3, 30, 31].
- Testing for kidney damage (albuminuria). In adults and children, a first morning sample is preferred for assessing albuminuria [3]. In adults, quantitative urinary albumin-creatinine ratio (uACR) is preferred as the most sensitive test [3]. Importantly, urinary albumin is in the process of being standardized analytically, which should ultimately facilitate worldwide uACR standardization [32]. In children, both protein-creatinine ratio (uPCR) and uACR should be tested in order to assess tubular proteinuria [3]. Semiquantitative albuminuria testing allows for flexibility for point of care or home-based testing [33]. Semiquantitative or qualitative screening tests should be positive in > 85% of individuals with quantitative uACR 30 mg/g or more to be useful [34]. In resource-constrained settings, urine protein dipstick testing may be used with a threshold of +2 proteinuria or greater to reduce false positive results for repeat confirmatory testing [35].
In specific populations, the following can be considered:
- Testing for hematuria is notable as the forgotten risk factor in recent clinical practice guidelines. It is particularly important for those at risk for glomerular disease, particularly IgA nephropathy [36].
- Baseline imaging in groups with signs or symptoms of structural abnormalities (e.g. pain and hematuria) to evaluate for kidney masses, cysts, stones, hydronephrosis or urinary retention is important. Antenatal ultrasound can detect hydronephrosis and other CAKUT.
- With increasing availability of genetic testing, family cascade CKD testing is indicated when there is a known genetic risk of kidney disease [37].
- In those who have an occupational risk of developing kidney disease, kidney testing should be offered as part of occupational health programs.
- Those who donate kidneys should be included in a post-donation surveillance program to assess kidney health over the long-term [38].
| Potential Benefits of Early Detection | ▴Top |
Screening for CKD fits with many of the World Health Organization’s Wilson-Jungner principles. Early stage CKD is asymptomatic and effective treatments, including lifestyle modification, interdisciplinary care and pharmacologic interventions are established [2, 3, 30, 35]. WHO essential medicines that improve CKD outcomes should be widely available, including ACE inhibitors, angiotensin receptor blockers, statins and sodium glucose co-transporter-2 inhibitors (SGLT2i) [2, 39]. SGLT2i alone are estimated to decrease the risk of CKD progression by 37% in people with and without diabetes [40]. For a 50-year-old person with albuminuria and non-diabetic CKD, this could extend their future period of healthy kidney function from 9.6 to 17 years [41]. These essential medicines reduce progression to more advanced CKD stages and limit cardiovascular hospitalization to provide short-term cost-effectiveness, especially for LICs. Where available and affordable the range of new paradigm-shifting medications to slow CKD progression also includes glucagon-like peptide-1 receptor antagonists, non-steroidal mineralocorticoid receptor antagonists, endothelin receptor antagonists and specific disease-modifying drugs (e.g. complement-inhibitors) that herald an exciting new era for nephrology.
Considering the significant healthcare costs associated with CKD, particularly hospitalization and kidney failure, effective preventive measures offer clear economic benefits for both high- and low-income countries. CKD confers enormous costs to the individual, their families, healthcare systems and governments worldwide. In the United States, CKD costs Medicare over US$ 85 billion annually [13]. In many high- and middle-income countries, 2-4% of the health budget is spent on kidney failure care alone. In Europe, healthcare costs associated with CKD are higher than those associated with cancer or diabetes [42]. Reducing the burden of kidney care worldwide will also have profound environmental effects, as it will save water and plastic waste, especially associated with dialysis [43]. On an individual level, CKD costs are frequently catastrophic, particularly in LICs and LMICs, where the individual largely bears the burden of payment. Only 13% of LICs and 19% of LMICs cover the costs of KFRT for adults [15]. CKD causes 188 million people in low and lower-middle-income countries annually to be faced with catastrophic healthcare expenditures [44].
The most widely cited and studied incremental cost effectiveness ratio (ICER) threshold to assess screening is US$ < 50,000 per quality-adjusted life year (QALY) [45]. If the prevalence of CKD is high, a population-wide screening strategy should be considered in HIC [33, 46]. For example, in the United States, a recent Markov simulation model of population-wide screening for CKD, which included appropriate SGLT2i treatment added to standard of care ACE inhibitors or angiotensin receptor blockers for adults age 35 to 75 years old with albuminuria, concluded that screening to identify CKD would be cost-effective [46]. In addition, an analysis of a home-based general population semiquantitative albuminuria screening in Holland was also found to be cost effective [33]. Case finding to detect CKD in higher risk groups rather than mass or general population screening will reduce costs and other harms whilst increasing the true positive rate of the screening tests [3, 35, 45]. An alternate ICER threshold proposed by WHO is < 1-3 times the ratio of the gross domestic product per capita income per QALY can be used to assess case finding approaches in LIC and LMIC [45]. The recommended tests for detecting kidney disease are low-cost and minimally invasive, facilitating their administration across diverse settings. Basic testing of eGFR and urinary ACR are widely available and using urine dipstick testing where quantitative proteinuria testing is unavailable or unaffordable will drastically reduce testing costs [31].
If coupled with effective intervention, early identification of people with kidney disease will benefit the individual, the health care system, governments and the economy [44]. Health and quality of life benefits for the individual would lead to improved productivity, especially in the young with more working years ahead, and to developmental/educational improvements in children and young adults. Individuals would face less catastrophic health expenses, governments and healthcare systems will save money not only on CKD care, but also on cardiovascular disease costs, and economies will benefit from more worker participation. This is especially crucial for lower-income countries, where the greatest burden of CKD exists and is cruelly coupled with the lowest ability for governments and individuals to afford kidney care.
| Challenges and Solutions for Implementation | ▴Top |
Structural barriers to widespread identification and treatment of people with CKD include cost, reliability of testing and lack of health information systems to track CKD burden. These are underpinned by a lack of relevant government and healthcare policy, low healthcare professional knowledge and implementation, poor general population perceived kidney disease risk and low patient CKD awareness. Solutions for implementing effective interventions include tying CKD identification to existing screening programs, educating the public and primary care professionals and leveraging non-governmental organization (NGO) joint advocacy programs to focus health policy agendas on kidney disease. Any solutions must balance the potential benefits and harms of screening and case-finding programs. Ethical implications for consideration include the availability of resources (such as health care workers and medicines), the affordability of testing and treatment, false positives or negatives and anxiety for patients and their families [47].
Screening and case-finding programs require workforce capacity, health information systems, reliable testing equipment and equitable access to medical care, medicines, vaccines and medical technologies. Primary care is at the front lines of the battle to protect kidney health, particularly in low and lower middle-income countries. The tiny nephrology workforce, with a median global prevalence of 11.8 nephrologists per million population and an 80-fold difference between LICs and HICs, is inadequate to detect and manage the vast majority of CKD [23]. As for other chronic diseases, primary care clinicians and other frontline health workers are foundational to early detection of CKD [48]. Testing must be affordable, simple and practical, with point-of-care creatinine testing and urine dipsticks useful in resource-limited settings [31]. Educational efforts directed at primary care clinicians are key to integrating CKD detection into routine care, despite constrained time and resources [49-51]. Automated clinical decision support could leverage electronic health records to identify people with CKD or at high-risk of CKD and recommend appropriate actions to clinicians (Fig. 2).
Currently, few countries have CKD registries, limiting our ability to highlight the disease burden to governments. Knowledge of CKD burden assists in prioritizing kidney health needs, which should then progressively expand to encompass the full spectrum of kidney care [52]. A global survey revealed only a quarter of the countries (41/162) had a nation specific CKD strategy and fewer than a third (48/162) recognized CKD as a public health priority [23]. WHO’s recognition of CKD as a major driver of NCD mortality would be impactful in increasing awareness, improving local surveillance and monitoring to implement clinical practice guidelines and improving resource allocation [2].
Programs for the early detection of CKD will require extensive coordination and engagement of stakeholders, including governments, health systems and insurers. International and national kidney organizations, such as the International Society of Nephrology (ISN), already advocate to the WHO and individual governments for the prioritization of kidney disease. We must continue this work, collaborating to streamline early detection program planning and implementation. Connection to existing community interventions (e.g. cardiovascular disease prevention) in LMICs and HICs can decrease cost and maximize efficiencies by integrating into existing programs. Such programs will need to be adapted to the local context and can be held in a variety of settings, such as individual healthcare practices, hospitals, as well as regional or national healthcare facilities or as outreaches in rural communities. Depending on local regulations and resources, screening and case-finding can also take place outside of medical settings such as town halls, churches or markets. Volunteers in the community can also assist with community-based screening and case-finding efforts.
In conjunction with reorienting the clinical practice of health care professionals to a greater focus on timely detection of CKD, we must focus on general population perceived risk education and health promotion activities, as well as education programs aimed at patient awareness and empowerment. General population awareness of kidney disease is poor, with 9 out of 10 people with CKD unaware they are affected [53]. Coverage of kidney disease is missing from the mainstream conversation, with an analysis of lay press showing kidney disease was 11-times under-represented in discussed compared to the actual cause of death [54]. A number of national and international organizations have developed public-facing quizzes on risk of kidney disease, supported by a regional study that showed socially vulnerable patients with hypertension do not understand their kidney risks [21, 55-57]. Online and direct education for healthcare professionals can improve consumer health literacy. Patient activation, engagement, and shared decision-making are downstream impacts of awareness. Awareness education is nuanced for CKD, including detection and risk stratification to inform and empower rather than frighten regarding the timing and extent of interventions (Supplementary Material 1, wjnu.elmerpub.com) [4, 27, 57]. Getting the balance right will optimize self-efficacy and patient, family and caregiver engagement.
| Conclusion: A Call to Action | ▴Top |
We call on all healthcare professionals to check the kidney health of their patients at risk of kidney disease. In tandem, we must work with public health organizations to improve the general population’s perceived risk of kidney disease and empower people at risk to seek kidney health checks. To ensure this change can be delivered, we must work with healthcare systems, governments and the WHO to prioritize kidney disease and create effective and efficient early detection programs for kidney disease. Only then will the paradigm-shifting benefits of lifestyle change and pharmacologic treatments translate to better kidney and overall health for people all around the world.
| Supplementary Material | ▴Top |
Suppl 1. Person perspectives on CKD awareness, detection and treatment from the literature.
Acknowledgments
We thank Valerie A. Luyckx, Marcello Tonelli, Ifeoma Ulasi, Vivekanand Jha, Marina Wainstein, Siddiq Anwar, Daniel O’Hara, Elliot K. Tannor, Jorge Cerda, Elena Cervantes, and Maria Carlota Gonzalez Bedat for their invaluable feedback on this paper.
Financial Disclosure
None to declare.
Conflict of Interest
All the authors declared no competing interests.
Author Contributions
All authors contributed to the writing and the revision of this paper.
Data Availability
The authors declare that data supporting the findings of this study are available within the article supplementary files.
| References | ▴Top |
- Ndumele CE, Neeland IJ, Tuttle KR, Chow SL, Mathew RO, Khan SS, Coresh J, et al. A synopsis of the evidence for the Science and Clinical Management of Cardiovascular-Kidney-Metabolic (CKM) Syndrome: a scientific statement from the American Heart Association. Circulation. 2023;148(20):1636-1664.
doi pubmed - Luyckx VA, Tuttle KR, Abdellatif D, Correa-Rotter R, Fung WWS, Haris A, Hsiao LL, et al. Mind the gap in kidney care: translating what we know into what we do. Kidney Int. 2024;105(3):406-417.
doi pubmed - Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2024 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. 2024;105(4S):S117-S314.
doi pubmed - Guha C, Lopez-Vargas P, Ju A, Gutman T, Scholes-Robertson NJ, Baumgart A, Wong G, et al. Patient needs and priorities for patient navigator programmes in chronic kidney disease: a workshop report. BMJ Open. 2020;10(11):e040617.
doi pubmed - G. B. D. Chronic Kidney Disease Collaboration. Global, regional, and national burden of chronic kidney disease, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2020;395(10225):709-733.
doi pubmed - Cojuc-Konigsberg G, Guijosa A, Moscona-Nissan A, Nordmann-Gomes A, Canaviri-Flores VA, Braverman-Poyastro A, de la Fuente-Ramirez R, et al. Representation of low- and middle-income countries in CKD drug trials: a systematic review. Am J Kidney Dis. 2025;85(1):55-66.e51.
doi pubmed - Hsiao LL, Shah KM, Liew A, Abdellatif D, Balducci A, Haris A, Kumaraswami LA, et al. Kidney health for all: preparedness for the unexpected in supporting the vulnerable. Kidney Int. 2023;103(3):436-443.
doi pubmed - Francis A, Harhay MN, Ong ACM, Tummalapalli SL, Ortiz A, Fogo AB, Fliser D, et al. Chronic kidney disease and the global public health agenda: an international consensus. Nat Rev Nephrol. 2024;20(7):473-485.
doi pubmed - Foreman KJ, Marquez N, Dolgert A, Fukutaki K, Fullman N, McGaughey M, Pletcher MA, et al. Forecasting life expectancy, years of life lost, and all-cause and cause-specific mortality for 250 causes of death: reference and alternative scenarios for 2016-40 for 195 countries and territories. Lancet. 2018;392(10159):2052-2090.
doi pubmed - Viggiano D, Wagner CA, Martino G, Nedergaard M, Zoccali C, Unwin R, Capasso G. Mechanisms of cognitive dysfunction in CKD. Nat Rev Nephrol. 2020;16(8):452-469.
doi pubmed - Chen K, Didsbury M, van Zwieten A, Howell M, Kim S, Tong A, Howard K, et al. Neurocognitive and Educational Outcomes in Children and Adolescents with CKD: A Systematic Review and Meta-Analysis. Clin J Am Soc Nephrol. 2018;13(3):387-397.
doi pubmed - Francis A, Didsbury MS, van Zwieten A, Chen K, James LJ, Kim S, Howard K, et al. Quality of life of children and adolescents with chronic kidney disease: a cross-sectional study. Arch Dis Child. 2019;104(2):134-140.
doi pubmed - United States Renal Data System. 2023 USRDS Annual Data Report: Epidemiology of kidney disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD. 2023.
- Liyanage T, Ninomiya T, Jha V, Neal B, Patrice HM, Okpechi I, Zhao MH, et al. Worldwide access to treatment for end-stage kidney disease: a systematic review. Lancet. 2015;385(9981):1975-1982.
doi pubmed - Bello AK, Levin A, Tonelli M, Okpechi IG, Feehally J, Harris D, Jindal K, et al. Assessment of global kidney health care status. JAMA. 2017;317(18):1864-1881.
doi pubmed - Ortiz A, Wanner C, Gansevoort R, Council ERA. Chronic kidney disease as cardiovascular risk factor in routine clinical practice: a position statement by the Council of the European Renal Association. Nephrol Dial Transplant. 2023;38(3):527-531.
doi pubmed - Johnson RJ, Wesseling C, Newman LS. Chronic kidney disease of unknown cause in agricultural communities. N Engl J Med. 2019;380(19):1843-1852.
doi pubmed - McCulloch M, Luyckx VA, Cullis B, Davies SJ, Finkelstein FO, Yap HK, Feehally J, et al. Challenges of access to kidney care for children in low-resource settings. Nat Rev Nephrol. 2021;17(1):33-45.
doi pubmed - Stanifer JW, Muiru A, Jafar TH, Patel UD. Chronic kidney disease in low- and middle-income countries. Nephrol Dial Transplant. 2016;31(6):868-874.
doi pubmed - Levey AS, Schoolwerth AC, Burrows NR, Williams DE, Stith KR, McClellan W, Centers for Disease C, et al. Comprehensive public health strategies for preventing the development, progression, and complications of CKD: report of an expert panel convened by the Centers for Disease Control and Prevention. Am J Kidney Dis. 2009;53(3):522-535.
doi pubmed - Nephrology ISo. World Kidney Day. 2025. Available from: https://www.worldkidneyday.org/about-kidney-health/.
- Lloyd-Jones DM, Allen NB, Anderson CAM, Black T, Brewer LC, Foraker RE, Grandner MA, et al. Life's essential 8: updating and enhancing the American Heart Association's Construct of Cardiovascular Health: a presidential advisory from the American Heart Association. Circulation. 2022;146(5):e18-e43.
doi pubmed - Bello AK, Okpechi IG, Levin A, Ye F, Damster S, Arruebo S, Donner JA, et al. An update on the global disparities in kidney disease burden and care across world countries and regions. Lancet Glob Health. 2024;12(3):e382-e395.
doi pubmed - Ferre S, Storfer-Isser A, Kinderknecht K, Montgomery E, Godwin M, Andrews A, Dunning S, et al. Fulfillment and validity of the kidney health evaluation measure for people with diabetes. Mayo Clin Proc Innov Qual Outcomes. 2023;7(5):382-391.
doi pubmed - Alfego D, Ennis J, Gillespie B, Lewis MJ, Montgomery E, Ferre S, Vassalotti JA, et al. Chronic kidney disease testing among at-risk adults in the U.S. remains low: real-world evidence from a national laboratory database. Diabetes Care. 2021;44(9):2025-2032.
doi pubmed - Stempniewicz N, Vassalotti JA, Cuddeback JK, Ciemins E, Storfer-Isser A, Sang Y, Matsushita K, et al. Chronic kidney disease testing among primary care patients with type 2 diabetes across 24 U.S. Health Care Organizations. Diabetes Care. 2021;44(9):2000-2009.
doi pubmed - Kushner PR, DeMeis J, Stevens P, Gjurovic AM, Malvolti E, Tangri N. Patient and clinician perspectives: to create a better future for chronic kidney disease, we need to talk about our kidneys. Adv Ther. 2024;41(4):1318-1324.
doi pubmed - Farrell DR, Vassalotti JA. Screening, identifying, and treating chronic kidney disease: why, who, when, how, and what? BMC Nephrol. 2024;25(1):34.
doi pubmed - Tuttle KR. CKD screening for better kidney health: Why? Who? How? When? Nephrol Dial Transplant. 2024;39(10):1537-1539.
doi pubmed - Shlipak MG, Tummalapalli SL, Boulware LE, Grams ME, Ix JH, Jha V, Kengne AP, et al. The case for early identification and intervention of chronic kidney disease: conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int. 2021;99(1):34-47.
doi pubmed - Tummalapalli SL, Shlipak MG, Damster S, Jha V, Malik C, Levin A, Johnson DW, et al. Availability and affordability of kidney health laboratory tests around the globe. Am J Nephrol. 2020;51(12):959-965.
doi pubmed - Seegmiller JC, Bachmann LM. Urine albumin measurements in clinical diagnostics. Clin Chem. 2024;70(2):382-391.
doi pubmed - van Mil D, Kieneker LM, Heerspink HJL, Gansevoort RT. Screening for chronic kidney disease: change of perspective and novel developments. Curr Opin Nephrol Hypertens. 2024;33(6):583-592.
doi pubmed - Sacks DB, Arnold M, Bakris GL, Bruns DE, Horvath AR, Lernmark A, Metzger BE, et al. Guidelines and recommendations for laboratory analysis in the diagnosis and management of diabetes mellitus. Clin Chem. 2023;69(8):808-868.
doi pubmed - Tonelli M, Dickinson JA. Early detection of CKD: implications for low-income, middle-income, and high-income countries. J Am Soc Nephrol. 2020;31(9):1931-1940.
doi pubmed - Moreno JA, Martin-Cleary C, Gutierrez E, Rubio-Navarro A, Ortiz A, Praga M, Egido J. Haematuria: the forgotten CKD factor? Nephrol Dial Transplant. 2012;27(1):28-34.
doi pubmed - Franceschini N, Feldman DL, Berg JS, Besse W, Chang AR, Dahl NK, Gbadegesin R, et al. Advancing genetic testing in kidney diseases: report from a national kidney foundation working group. Am J Kidney Dis. 2024;84(6):751-766.
doi pubmed - Mjoen G, Holdaas H. Mid- and long-term health risks in living kidney donors. Ann Intern Med. 2018;169(4):265.
doi pubmed - Francis A, Abdul Hafidz MI, Ekrikpo UE, Chen T, Wijewickrama E, Tannor EK, Nakhoul G, et al. Barriers to accessing essential medicines for kidney disease in low- and lower middle-income countries|. Kidney Int. 2022;102(5):969-973.
doi pubmed - Nuffield Department of Population Health Renal Studies, Group, Sglt inhibitor Meta-Analysis Cardio-Renal Trialists' Consortium. Impact of diabetes on the effects of sodium glucose co-transporter-2 inhibitors on kidney outcomes: collaborative meta-analysis of large placebo-controlled trials. Lancet. 2022;400(10365):1788-1801.
doi pubmed - Vart P, Vaduganathan M, Jongs N, Remuzzi G, Wheeler DC, Hou FF, McCausland F, et al. Estimated lifetime benefit of combined RAAS and SGLT2 inhibitor therapy in patients with albuminuric CKD without diabetes. Clin J Am Soc Nephrol. 2022;17(12):1754-1762.
doi pubmed - Vanholder R, Annemans L, Brown E, Gansevoort R, Gout-Zwart JJ, Lameire N, Morton RL, et al. Reducing the costs of chronic kidney disease while delivering quality health care: a call to action. Nat Rev Nephrol. 2017;13(7):393-409.
doi pubmed - Berman-Parks N, Berman-Parks I, Gomez-Ruiz IA, Ardavin-Ituarte JM, Piccoli GB. Combining patient care and environmental protection: a pilot program recycling polyvinyl chloride from automated peritoneal dialysis waste. Kidney Int Rep. 2024;9(6):1908-1911.
doi pubmed - Essue BM, Laba M, Knaul F, Chu A, Minh HV, Nguyen TKP, Jan S. Economic burden of chronic ill health and injuries for households in low- and middle-income countries. In: Jamison DT, Gelband H, Horton S, Jha P, Laxminarayan R, Mock CN, Nugent R, eds. Disease control priorities: improving health and reducing poverty. 3rd ed. Washington (DC); 2017.
doi pubmed - Yeo SC, Wang H, Ang YG, Lim CK, Ooi XY. Cost-effectiveness of screening for chronic kidney disease in the general adult population: a systematic review. Clin Kidney J. 2024;17(1):sfad137.
doi pubmed - Cusick MM, Tisdale RL, Chertow GM, Owens DK, Goldhaber-Fiebert JD. Population-wide screening for chronic kidney disease: a cost-effectiveness analysis. Ann Intern Med. 2023;176(6):788-797.
doi pubmed - Yadla M, John P, Fong VK, Anandh U. Ethical issues related to early screening programs in low resource settings. Kidney Int Rep. 2024;9(8):2315-2319.
doi pubmed - Szczech LA, Stewart RC, Su HL, DeLoskey RJ, Astor BC, Fox CH, McCullough PA, et al. Primary care detection of chronic kidney disease in adults with type-2 diabetes: the ADD-CKD Study (awareness, detection and drug therapy in type 2 diabetes and chronic kidney disease). PLoS One. 2014;9(11):e110535.
doi pubmed - Vassalotti JA, Centor R, Turner BJ, Greer RC, Choi M, Sequist TD, National Kidney Foundation Kidney Disease Outcomes Quality I. Practical approach to detection and management of chronic kidney disease for the primary care clinician. Am J Med. 2016;129(2):153-162.e157.
doi pubmed - Thavarajah S, Knicely DH, Choi MJ. CKD for primary care practitioners: can we cut to the chase without too many shortcuts? Am J Kidney Dis. 2016;67(6):826-829.
doi pubmed - Vassalotti JA, Boucree SC. Integrating CKD into US primary care: bridging the knowledge and implementation gaps. Kidney Int Rep. 2022;7(3):389-396.
doi pubmed - Luyckx VA, Moosa MR. Priority setting as an ethical imperative in managing global dialysis access and improving kidney care. Semin Nephrol. 2021;41(3):230-241.
doi pubmed - CDC. Chronic Kidney Disease in the United States. 2023. Available from: https://www.cdc.gov/kidneydisease/publications-resources/CKD-national-facts.html.
- Ritchie H. Does the news reflect what we die from? 2019 [cited Sept 8, 2022]. Available from: https://ourworldindata.org/does-the-news-reflect-what-we-die-from.
- Boulware LE, Carson KA, Troll MU, Powe NR, Cooper LA. Perceived susceptibility to chronic kidney disease among high-risk patients seen in primary care practices. J Gen Intern Med. 2009;24(10):1123-1129.
doi pubmed - Foundation NK. Kidney Quiz. 2024. Available from: https://www.kidney.org/kidney-quiz/.
- Tuot DS, Crowley ST, Katz LA, Leung J, Alcantara-Cadillo DK, Ruser C, Talbot-Montgomery E, et al. Usability testing of the kidney score platform to enhance communication about kidney disease in primary care settings: qualitative think-aloud study. JMIR Form Res. 2022;6(9):e40001.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Nephrology and Urology is published by Elmer Press Inc.